Figures & data
Figure 1. Design and expression of CTB-3 × M2e-HA2. (A) Amino acid sequences of 3 M2e extracted from A/California/04/2009 (H1N1), A/Viet Nam/1204/2004 (H5N1) and A/Hong Kong/1/1968 (H3N2) influenza virus and the variable amino acids were underlined. (B) Highly centralized HA2 amino acid sequence. (C) Schematics of chimeric CTB-3 × M2e-HA2 and 3 × M2e-HA2 proteins. (D) Expression of CTB-3 × M2e-HA2 and 3 × M2e-HA2 proteins were verified by Western Blot using an anti-6 × His monoclonal antibody.
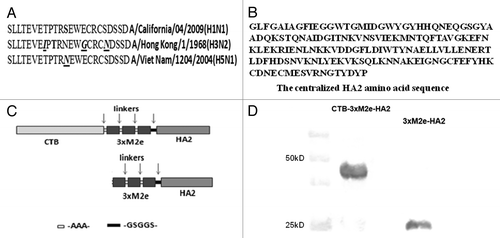
Figure 2. Evaluation of humoral and protective immunities by vaccine candidates. Mice were intranasally (i.n.) or intramuscularly (i.m.) primed with 10 µg recombinant protein, CTB-3 × M2e-HA2 or 3 × M2e-HA2. Boosters were given on days 14 and 28. Naïve, control mice received PBS (i.n.). Blood samples were collected on days 0, 14, 28, and 42. At 42 d post-immunization, all mice were challenged with 100 × LD50 PR8 influenza virus. Serum levels of 3 × M2e-HA2 specific (A) IgG, (B) IgG1, and (C) IgG2a antibodies as measured by ELISA are shown, ***P < 0.05. (D) Survival of mice challenged with 100 × LD50 PR8 influenza virus.
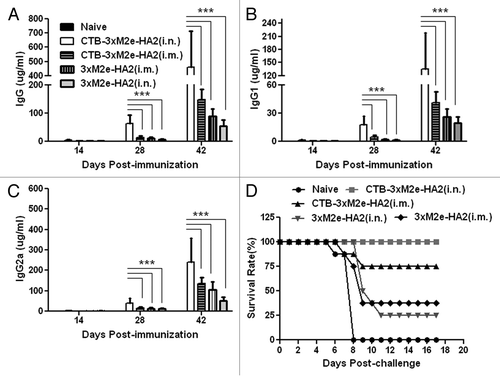
Figure 3. Mucosal immune responses in intranasally vaccinated mice. Mice in one group served as naïve controls, while mice in the other groups were intranasally primed at day 0 and boosted at days 14 and 28 with 10 µg CTB-3 × M2e-HA2 or 3 × M2e-HA2. Bronchoalveolar lavage fluid was collected on day 42 to measure anti-3xM2e-HA2 (A) IgA, (B) IgG, (C), IgG1, and (D) IgG2a concentrations by ELISA, ***P < 0.05.
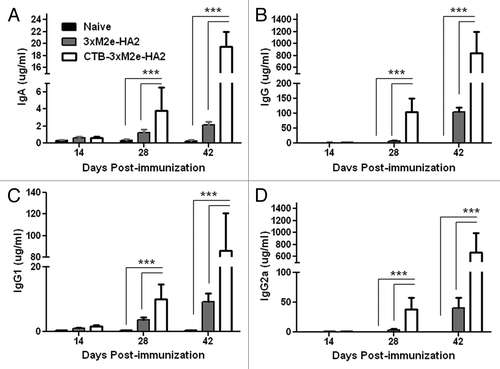
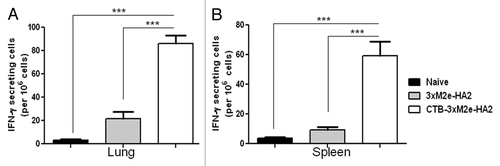
Figure 4. Testing broad protection against multiple influenza strains by CTB-3 × M2e-HA2. (A) Survival of mice immunized with CTB-3 × M2e-HA2 or CA09 in comparison to naïve mice after lethal challenge with 100 × LD50 CA09 influenza virus. (B) Lung viral loads in mice immunized with CTB-3 × M2e-HA2 or HK68 in comparison to naïve mice after challenge with 105 PFU HK68 influenza virus. (C) MDCK cells were infected with different viral strains at MOI = 0.1 to be used as source of viral HA2 and NP. Western Blot analysis was used to test if serum from mice immunized with CTB-3 × M2e-HA2 reacted with HA2 (about 26 kD) from infected MDCK cell lysates (top panel). A commercial, monoclonal antibody against NP was used to verify influenza viral infection of MDCK cells (middle panel). β-actin blots were used as loading controls.
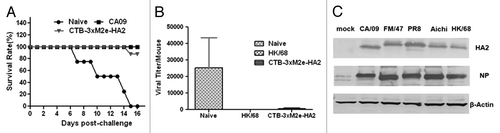
Figure 5. CTB-3 × M2e-HA2 antigen dose response. Mice were immunized once, twice, or thrice with CTB-3 × M2e-HA2 and challenged on day 42 after the first immunization with 100 × LD50 PR8 influenza virus.
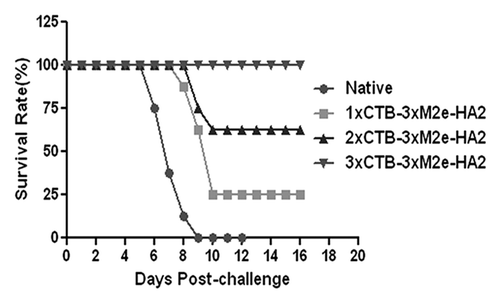
Figure 6. Evaluating the role of humoral immunity in protection against PR8 and CA09 influenza viruses. (A) Mice were i.p. injected with serum collected from naïve mice or mice immunized with CTB-3 × M2e-HA2, and then challenged with lethal doses of CA09 or PR8 influenza viruses. Clinical symptoms and survival were monitored and recorded. (B) B cell depleted mice were immunized with CTB-3 × M2e-HA2 or 3 × M2e-HA2, and then challenged with 100 × LD50 PR8 influenza virus.
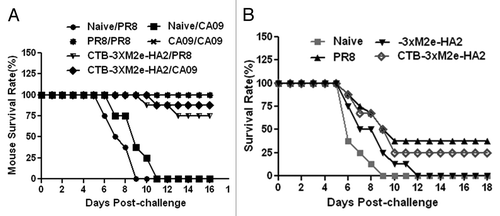
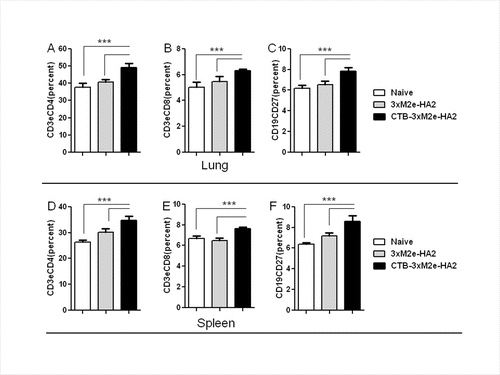
Figure 7. T cell and memory B cell immune responses elicited by vaccination with CTB-3 × M2e-HA2. After mice were immunized, splenocytes and lung cells were collected and CD4, CD8 T, and memory B cell percentages were analyzed by flow cytometry, ***P < 0.05. (A) Percentage of CD3e and CD4 double positive cell in lungs. (B) Percentage of CD3e and CD8 double positive T cells in lungs. (C) Percentage of CD19 and CD27 double positive cell in lungs. (D) Percentage of CD3e and CD4 double positive cell in spleens. (E) Percentage of CD3e and CD8 double positive T cells in spleens. (F) Percentage of CD19 and CD27 double positive cell in spleens.