Figures & data
Table 1. Study groups and vaccine formulations
Table 2. Study population demographics
Table 3. Geometric mean titers (GMTs) and geometric mean ratios (GMRs) at baseline (day 1), 1 wk (day 8), and 3 wk (day 22) after vaccination (95% CI)
Table 4. Immunogenicity analyses against A/H3N2, A/H1N1, and B strain vaccine antigens according to the European (CHMP) licensure criteria for influenza vaccines for older adults
Figure 1. Percentages of subjects experiencing any solicited local and systemic adverse reactions within 1 wk (days 1−7) of vaccination.
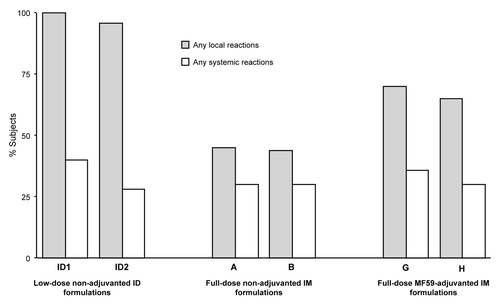
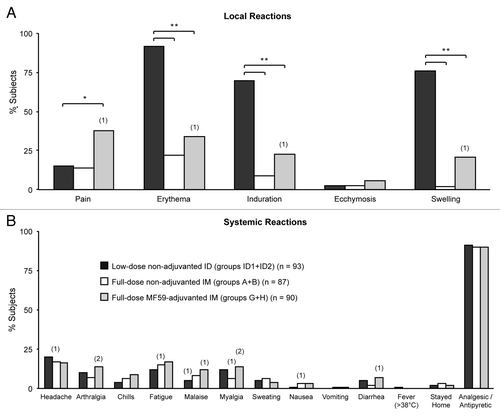
Figure 2. Percentages of subjects experiencing solicited local (A) and systemic (B) adverse reactions following vaccination. Bars show percentages of subjects experiencing reactions after vaccination in the low-dose non-adjuvanted ID groups (groups ID1+ID2) (black bars); in the full-dose non-adjuvanted IM groups (groups A+B) (open bars); and in the full-dose MF59-adjuvanted IM groups (groups G+H) (gray bars). Numbers in parentheses above bars indicate numbers of subjects experiencing severe reactions. Asterisks indicate significant differences between groups: ∗ 2 sided P < 0.01; ∗∗ 2 sided P < 0.0001.