Figures & data
Table 1. Pneumococcal OPA GMTs and subjects achieving OPA titers ≥ LLOQ by age group in the Japanese study (evaluable immunogenicity population)
Table 2. Serotype pneumococcal OPA GMTs for age groups 50–64 y and ≥65 y across studies (evaluable immunogenicity population)
Figure 1. Scatter plots and regression analysis between prevaccination diphtheria toxoid IgG concentrations and postvaccination pneumococcal OPA titers by serotype and age group (evaluable immunogenicity population). IgG, immunoglobulin G; OPA, opsonophagocytic activity.
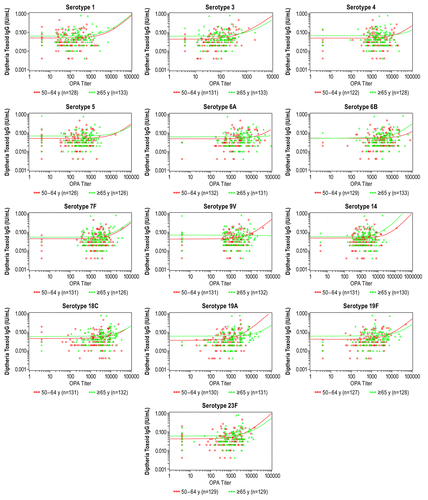
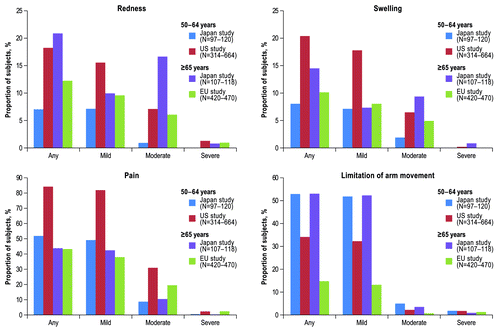
Figure 2. Subjects reporting local reactions within 14 d after vaccination by age group (safety population)*. *Severity of local reactions were categorized as follows: redness and swelling, mild = 2.5–5.0 cm, moderate = 5.1–10.0 cm, and severe >10.0 cm; pain, mild = awareness of symptom but easily tolerated, moderate = discomfort enough to cause interference with usual activity, and severe = incapacitating with inability to do usual activity; limitation of arm movement, mild = some limitation, moderate = unable to move above head but able to move above shoulder, and severe = unable to move above shoulder. Abbreviation: EU, European Union.