Figures & data
Figure 1. Serum antibody concentrations. Before and 1 mo after booster vaccination, serum antibody concentrations were assessed by ELISA for the indicated serotypes. (A) GMCs. (B) Rates of seroprotection, defined as ≥0.35 μg/mL.
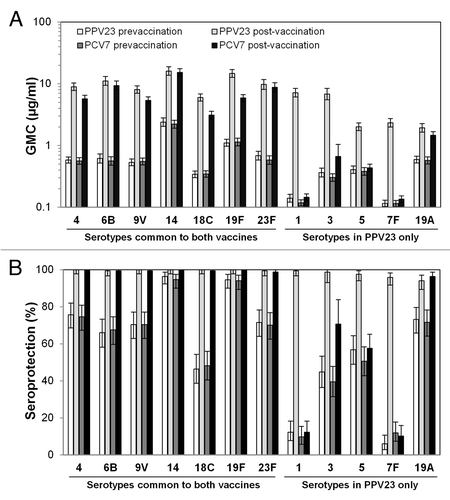
Figure 2. MOPA titers. Before and 1 mo after booster vaccination, functional antibody levels were assessed by MOPA for the indicated serotypes. (A) Geometric mean OIs. (B) Ratio of pre-vaccination geometric mean OI to post-vaccination geometric mean OI. In both panels, error bars indicate 95% confidence intervals. Values are shown for all subjects vaccinated according to the vaccine received.
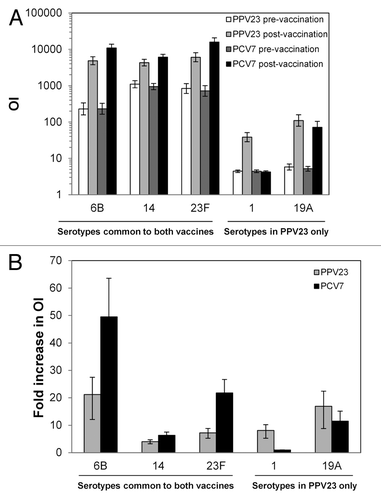
Table 1. Safety profiles between groups
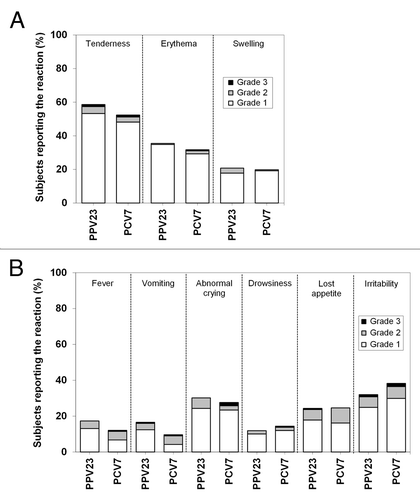
Figure 3. Solicited reactions. Solicited injection site (A) and systemic reactions (B) were assessed up to 7 d after vaccination. Solicited reactions were graded as follows: tenderness, 1 for a minor reaction when injection site is touched, 2 for child cries when injection site is touched, 3 for cries when injection site is moved or the movement of the injected limb is reduced; erythema and swelling, 1 for <2.5 cm in diameter, 2 for 2.5 to <5 cm in diameter, 3 for ≥5 cm in diameter; fever (axillary temperature), 1 for 38 °C to <38.5 °C, 2 for 38.5 °C to <39.5 °C, 3 for ≥39.5 °C; vomiting, 1 for 1 episode per 24 h, 2 for 2 to 5 episodes per 24 h, 3 for ≥6 episodes per 24 h; abnormal crying, 1 for <1 h, 2 for 1 to 3 h, 3 for >3 h; drowsiness, 1 for sleepier than usual or less interested in surroundings, 2 for not interested in surroundings or did not wake up for a feed/meal, 3 for sleeping most of the time or difficult to wake; loss of appetite, 1 for eating less than normal, 2 for missed 1 or 2 feeds/meals completely, 3 for refuses ≥3 feeds/meals or refuses most feeds/meals; irritability, 1 for easily consolable, 2 for requiring increased attention, 3 for inconsolable. Values are shown for all subjects vaccinated according to the vaccine received.