Figures & data
Figure 2. Antigen adsorbed on PRINT particles. Recombinant H3 HA was adsorbed on 1 × 1 μm and 1 × 3 μm cylinders (where first number is a diameter and second number is length in micrometers) and 1 × 1 × 10 μm, 2 × 2 × 2 μm, 2 × 2 × 6 μm rods (where first, second, and third number correspond to height, width, and length, in micrometers). Particles along with a 2 μg dose of soluble H3 HA protein were injected intramuscularly into BALB/c mice followed by IgG antibody level measurement on day 21 after injection.
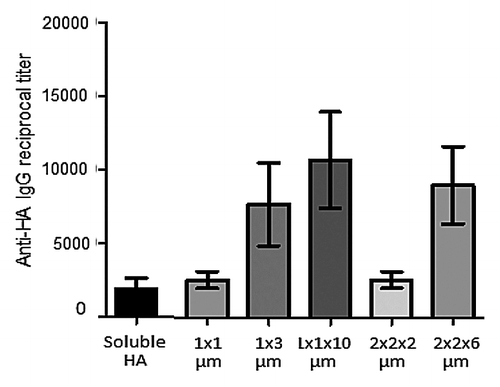
Figure 3. CSP antigen and TLR 7/8 adjuvant co-delivery using PRINT particles. 80 × 80 × 320 nm PLGA-DC Chol particles with a proprietary TLR 7/8 ligand encapsulated in the particle matrix and CSP protein adsorbed on the surface of the particle were injected intramuscularly into BALB/c mice. CSP, matched dose of soluble protein control; CSP+TLR7/8, matched dose of soluble protein control mixed with TLR7/8 ligand; PRINT CSP TLR7/8, PRINT particle designed for co-delivery of CSP and TLR7/8 ligand. (A) Anti-CSP IgG serum antibody levels were evaluated 21 d after a single injection. (B) Cellular responses were evaluated 7 d post-immunization for IFN-γ, IL-2, and TNFα by re-stimulating splenocytes with CSP antigen. Average % of cytokine-producing CD4+ and CD8+ cells is shown. Response for PRINT particle containing CSP alone without adjuvant was at baseline and is not shown.
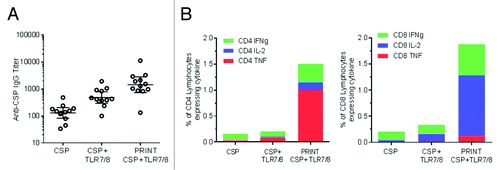
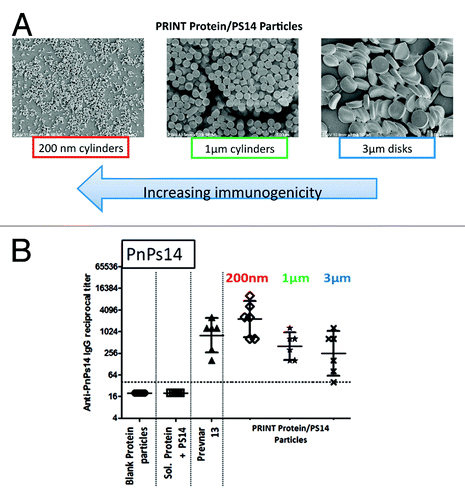
Figure 4. Anti-polysaccharide ELISA immune responses to pneumococcal polysaccharide type 14 (PnPs14). (A) PnPs14 was encapsulated in 0.2 × 0.2 μm, 1 × 1 μm cylinders (where first number is a diameter and second number is length in micrometers) and 1 × 3 μm disks (where first number is height and second number is a diameter, in micrometers) consisting of crosslinked chicken ovalbumin (OVA) protein matrix. (B) BALB/c mice were injected intramuscularly on days 1, 29, and 57. Anti-PnPs14 IgG levels were measured on day 71. Blank Protein particles, OVA protein particles without PnPs14; Sol. Protein+PS14, soluble mix of OVA protein and PnPs14; Prevnar 13®, commercial pneumococcal 13-valent conjugate vaccine; PRINT Protein/PS14 Particles, particles with PnPs14 encapsulated in OVA protein matrix, as described in (A).