Figures & data
Figure 1. Detection of serum and mucosal VP6-specific antibody responses following intranasal immunization. Endpoint titration of VP6-specific IgG (A) and IgA (B) antibodies in serum of individual mice (5 mice/group) after 2 intranasal immunizations with the combination vaccine containing rotavirus rVP6 and norovirus VLPs, each at a 10 µg dose. Control (ctrl) mice received only PBS. Mean titers of sera at study week 5 are shown. Error bars represent standard error of the means. (C) Endpoint titration of VP6-specific IgG and IgA antibodies in mucosal samples of experimental groups. Mean titers of groupwise pooled (5 mice/group) vaginal washes (VW) and fecal suspensions of at least 2 (2–4) independent experiments at week 5 are shown. (D) Characterization of different IgA forms in mucosal samples from immunized mice by immunoblot analysis under non-reducing conditions. Polymeric IgA (pIgA) forms including secretory IgA (SIgA) were confirmed upon immunodetection with goat anti-mouse IgA. Molecular weight markers (in kDa) are indicated with arrows.
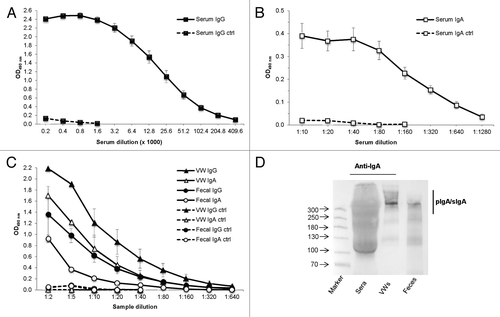
Figure 2. Inhibition activity of sera and vaginal washes of intranasally immunized mice against human rotavirus (RV) Wa (homologous VP6 to the immunizing rVP6 protein) or rhesus RV (RRV) using an ELISA-based antigen reduction neutralization assay. Mice were immunized with the combination vaccine containing RV rVP6 and norovirus virus-like particles. Control mice received no immunogen. Sera of RV-seropositive (RV+) and RV-seronegative (RV–) human donors were used as assay controls. The reciprocal of the sample dilution that generated >60% reduction in virus infectivity was considered its titer. If the highest dilution (1:10) did not yield neutralization of >60%, a titer of 5 was assigned as the neutralization titer of the sample. Results of study week 5 are represented as the geometric mean neutralizing titer of at least 2 independent experiments, each done in duplicate, with standard errors. NT, not tested.
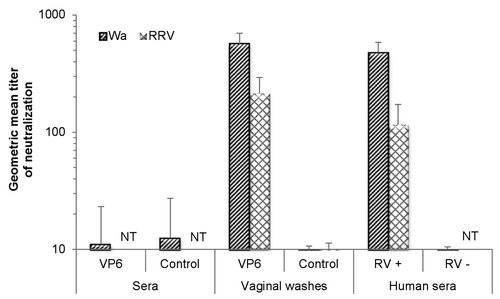
Figure 3. VP6-specific mucosal IgA mediates RV inhibition in vitro. (A) Blocking of the inhibition of human rotavirus (RV) Wa and rhesus RV (RRV) infection in vitro by VP6-specific antibodies of mice immunized intranasally with the combination vaccine containing RV rVP6 and norovirus (NoV) virus-like particles (VLPs). Control (Ctrl) mice received no immunogen. Vaginal wash (VW) samples from immunized mice were preincubated with rVP6 protein or NoV GII-4 VLPs prior the neutralization assay or left untreated. (B) IgA antibodies mediate RV Wa and RRV inhibition in vitro. IgA was depleted (–IgA) from VWs of immunized mice prior to neutralization assay, resulting in reduced inhibitory activity compared with untreated VWs. The dashed line indicates the 60% cut-off for reduction in virus infectivity. Results of at least 2 independent experiments, each done in duplicate, with standard errors are shown.
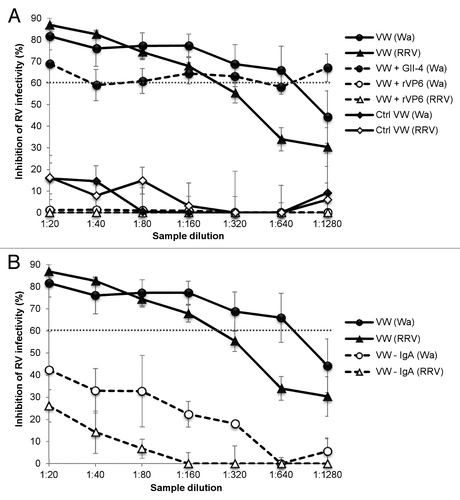
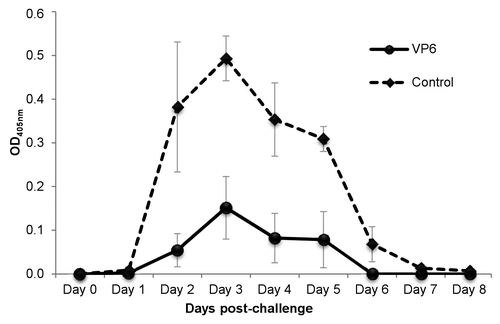
Figure 4. Effect of intranasal immunization with candidate vaccine on rotavirus shedding. Groups of mice were immunized intranasally twice with the candidate vaccine containing rotavirus (RV) rVP6 and norovirus virus-like particles, each at a 10 µg dose. Three weeks after the second dose mice were challenged orally with EDIMwt (100-fold DD50) and the quantity of RV antigen shed in fecal samples was determined up to 8 d post-challenge by ELISA. Each point represents the daily average of a group of mice (4 mice/group) with standard error of the means.