Figures & data
Table 1. Summary of injection site reactions and body weights at day 63 (sacrifice of main group) and mean food conversion efficiencies at day 77 (total study period)
Table 2. Summary of main haematology and clinical chemistry data
Figure 1. PRP- specific IgG antibodies in serum after vaccination. Rats were vaccinated with 3 doses of a licensed Hib vaccine (square) and the test Hib vaccine (triangle) on day 0, 28, and 56. As a negative control a group of rats was vaccinated using a placebo (circle). The level of PRP- specific IgG antibodies were measured in a rat-anti-PRP ELISA at OD450
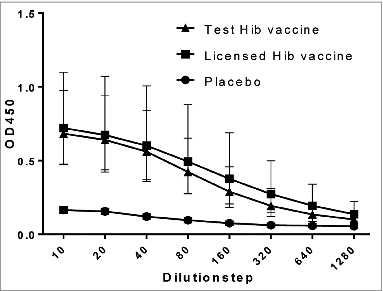
Table 3. Study design of the repeated dose toxicity study with Haemophilus influenza b vaccine
