Figures & data
Figure 1. Flow of participants HPV-001: NCT00689741; HPV-007: NCT00120848; HPV-023: NCT00518336. ATP = according-to-protocol. The ATP cohort for primary analysis of efficacy included all women for whom differential treatment effect on efficacy was likely (i.e., those meeting all eligibility criteria in HPV-001, HPV-007 and HPV-023), complying with the procedures defined in the respective study protocol, and for whom data concerning efficacy endpoint measures were available. The ATP immunogenicity cohort included all evaluable women (i.e., those meeting all eligibility criteria, complying with the procedures defined in the protocol, and fulfilling requirements for analysis) for whom data concerning immunogenicity were available. M0 = Month 0 = time of randomization; M18 = Month 18 (18 mo after the first dose of vaccine); M33 = Month 33 (33 mo after the first dose); M76 = Month 76 (76 mo after the first dose); M77 = Month 77 (77 mo after the first dose); M113 = Month 113 (113 mo after the first dose).
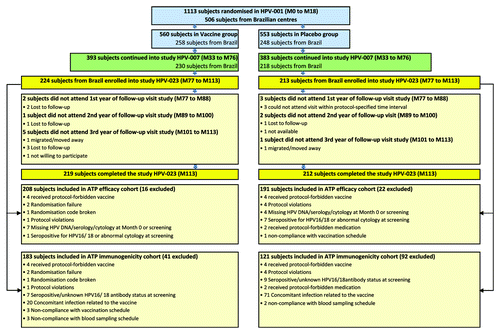
Table 1. Vaccine efficacy against infection (incident and persistent) and cyto-histopathological abnormalities associated with HPV-16/18
Figure 2. Reverse cumulative distribution curves for HPV-16/18 incident infection (A) and HPV-16/18 6-mo persistent infection (B) in cervical samples (ATP efficacy cohort). Combined analysis of initial and follow-up studies (HPV-001/007/023). Vaccine = HPV-16/18 vaccine group. Placebo = placebo group. VE = vaccine efficacy, with 95% confidence interval.
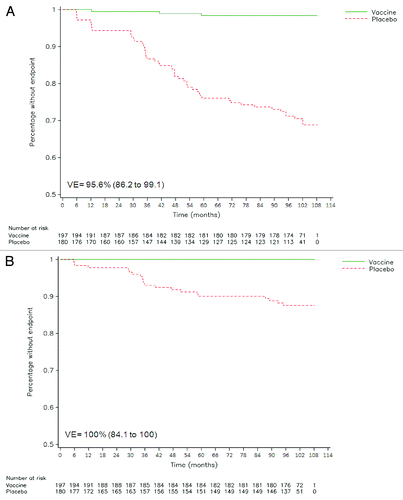
Table 2. Vaccine efficacy against infection (incident and persistent) and cyto-histopathological abnormalities associated with any oncogenic HPV type§
Figure 3. Seropositivity rates and geometric mean titers for anti-HPV-16 (A) and anti-HPV-18 (B) antibodies, measured by ELISA (ATP immunogenicity cohort). ATP immunogenicity cohort = women who met all eligibility criteria (all had received 3 doses of vaccine or placebo), complied with study procedures in the current and preceding studies, and had data available for at least one vaccine antibody blood sample. Data are shown for the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies. Histogram bars show the GMT and corresponding 95% Confidence intervals (CI). ELISA = enzyme-linked immunosorbent assay; HPV = HPV-16/18 vaccine group; Placebo = placebo group; PRE = pre-vaccination; PII = post dose II; PIII = post dose III; M = Month. EL.U/mL = ELISA units/mL. Figures above the bars are the seropositivity rates for the corresponding timepoint. Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-008, NCT00122681) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-008 were 29·8 EL.U/mL (95% CI: [28·5 to 31·0]) for HPV-16 and 22·6 EL.U/mL (95% CI: [21·6 to 23·6]) for HPV-18; measured by ELISA.Citation16
![Figure 3. Seropositivity rates and geometric mean titers for anti-HPV-16 (A) and anti-HPV-18 (B) antibodies, measured by ELISA (ATP immunogenicity cohort). ATP immunogenicity cohort = women who met all eligibility criteria (all had received 3 doses of vaccine or placebo), complied with study procedures in the current and preceding studies, and had data available for at least one vaccine antibody blood sample. Data are shown for the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies. Histogram bars show the GMT and corresponding 95% Confidence intervals (CI). ELISA = enzyme-linked immunosorbent assay; HPV = HPV-16/18 vaccine group; Placebo = placebo group; PRE = pre-vaccination; PII = post dose II; PIII = post dose III; M = Month. EL.U/mL = ELISA units/mL. Figures above the bars are the seropositivity rates for the corresponding timepoint. Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-008, NCT00122681) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-008 were 29·8 EL.U/mL (95% CI: [28·5 to 31·0]) for HPV-16 and 22·6 EL.U/mL (95% CI: [21·6 to 23·6]) for HPV-18; measured by ELISA.Citation16](/cms/asset/68c22677-6605-4d91-a5ff-9efb44f81c82/khvi_a_10929532_f0003.gif)
Figure 4. Seropositivity rates and geometric mean titers for (A) anti-HPV-16 and (B) anti-HPV-18 antibodies, measured by PBNA (ATP immunogenicity cohort). ATP cohort for immunogenicity = women who met all eligibility criteria (all had received 3 doses of vaccine or placebo), complied with study procedures in the current and preceding studies, and had data available for at least one vaccine antibody blood sample. Data are shown for a subset of the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies. Histogram bars show the GMT and corresponding 95% Confidence intervals (CI). PBNA = Pseudovirion-Based Neutralisation Assay; PRE = pre-vaccination; PII = post dose II; PIII = post dose III; M = Month. Figures above the bars are the seropositivity rates for the corresponding timepoint. Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-010, NCT00423046) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-010 were 180·1 ED50 (95% CI: [153·3 to 211·4]) for HPV-16 and 137·3 ED50 (95% CI: [112·2 to 168·0]) for HPV-18; measured by PBNA).Citation24
![Figure 4. Seropositivity rates and geometric mean titers for (A) anti-HPV-16 and (B) anti-HPV-18 antibodies, measured by PBNA (ATP immunogenicity cohort). ATP cohort for immunogenicity = women who met all eligibility criteria (all had received 3 doses of vaccine or placebo), complied with study procedures in the current and preceding studies, and had data available for at least one vaccine antibody blood sample. Data are shown for a subset of the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies. Histogram bars show the GMT and corresponding 95% Confidence intervals (CI). PBNA = Pseudovirion-Based Neutralisation Assay; PRE = pre-vaccination; PII = post dose II; PIII = post dose III; M = Month. Figures above the bars are the seropositivity rates for the corresponding timepoint. Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-010, NCT00423046) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-010 were 180·1 ED50 (95% CI: [153·3 to 211·4]) for HPV-16 and 137·3 ED50 (95% CI: [112·2 to 168·0]) for HPV-18; measured by PBNA).Citation24](/cms/asset/7d3e1bc5-993a-4aae-927e-d28d720271e1/khvi_a_10929532_f0004.gif)
Figure 5. Anti-HPV-16 antibody responses predicted by the modified power-law (A), piece-wise model (B), and their comparison (C), up to 20 y. GMT = geometric mean titer; EL.U/mL = ELISA units/mL; Natural infection = mean antibody titers associated with natural infection were obtained from women enrolled in a Phase III efficacy study (HPV-008, NCT00122681).Citation16
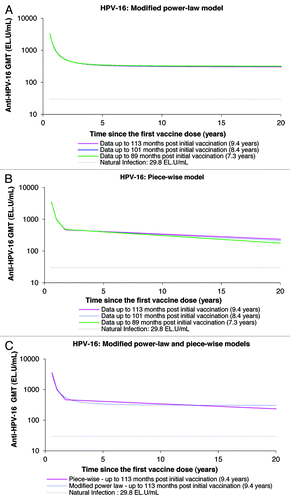
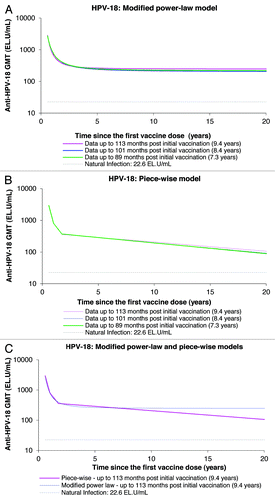
Figure 6. Anti-HPV-18 antibody responses predicted by the modified power-law (A), piece-wise model (B), and their comparison (C), up to 20 y. GMT = geometric mean titer; EL.U/mL = ELISA units/mL; Natural infection = mean antibody titers associated with natural infection were obtained from women enrolled in a Phase III efficacy study (HPV-008, NCT00122681).Citation16