Figures & data
Figure 1. DC development. This illustration summarizes the current model of the developmental pathways of both myeloid and plasmacytoid DCs. Dashed lines indicate pathways that are likely but not yet definitively shown to operate in DC development. In humans, equivalents of mouse MDP, CDP, and pre-DC have not been found. Cytokines that are important in each transition are indicated. Abbreviations: MPP, multi potential progenitor; CMP, common myeloid progenitor; CLP, common lymphoid progenitor; MDP, macrophage DC progenitor; CDP, common DC progenitor; pre-DC, circulating DC progenitor; pDC, plasmacytoid DC; mDC, myeloid DC; Mo-DC, monocyte-derived DC.
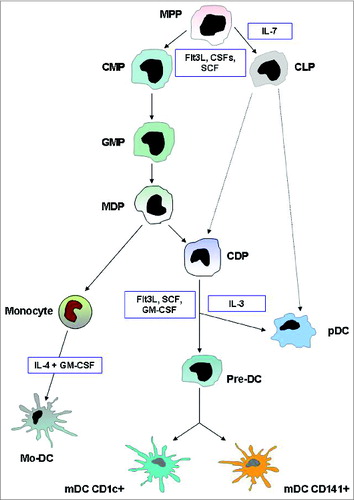
Figure 2. Immunophenotypic and functional hallmarks of DC subsets in blood and lymphoid tissue. (A) Both mDC and pDC cells are effective T-cell stimulators and direct the nature of Th responses, although mDC cells primarily induce Th1 differentiation, whereas pDC cells mainly promote a Th2 response. The archetypical antigen/cytokine of each subset is marked in bold. (B) Key cytokines involved in the DC-mediated polarization of naïve T cells into different T-cell subsets. Mature dendritic cells polarize naive Th0 cells into different Th effector cells through several signals: antigen presentation to the T-cell receptor, co-stimulatory signal and secretion of cytokines. Immature dendritic cells prime Th0 cells to make Treg cells. Abbreviations: pDC, plasmacytoid DC; mDC, myeloid DC; Lin, lineage markers; PRRs, pattern-recognition receptors; TLR, Toll like receptors Th, T helper cell; Treg, regulatory T cell; Ag, antigen, TCR, T-cell receptor; CTLA-4, cytotoxic T-lymphocyte antigen 4.
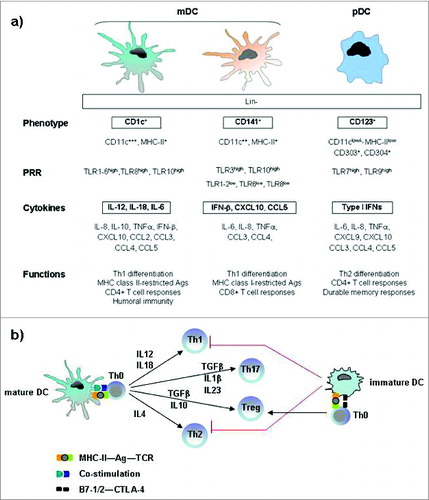
Figure 3. Tumor-altered DC function. Tumor-secreted factors can inhibit DC maturation. Immature DCs, displaying low NF-kB activation, low MHC class II and co-stimulatory molecule expression, are defective antigen presenting cells and induce T cell anergy and exhaustion. Tumor-derived factors can also induce the development of immunosuppressive regulatory DCs, which suppress T cell function through multiple mechanisms. More informations regarding the mechanisms by which tumors alter DC function and suppress host anti-tumor immunity are illustrated in the review of Hargadon.Citation84 Abbreviations: iDC, immature DC; MDC, mature DC; Reg DC, regulatory DC; Treg, regulatory T cell; PD-1L, programmed death 1 ligand.
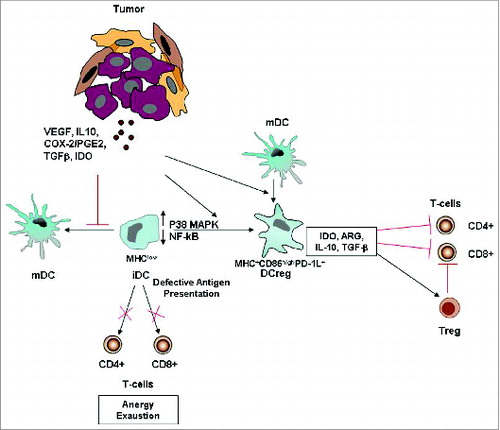
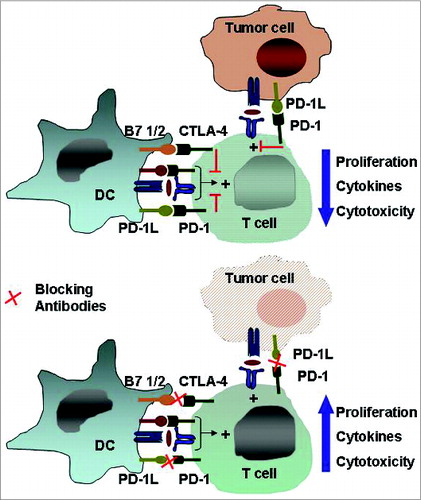
Figure 4. The immune checkpoints CTLA-4 and PD-1/PD-L1. Inhibitory receptors such as anti-cytotoxic T-lymphocyte antigen 4 and programmed death 1 expressed on tumor-specific T cells lead to compromised activation and suppressed effector functions such as proliferation, cytokine secretion, and tumor cell lysis. Modulating these receptors using monoclonal antibodies, an approach termed “immune checkpoint blockade,” has gained momentum as a new approach in cancer immunotherapy. Abbreviations: CTLA-4, cytotoxic T-lymphocyte antigen 4; PD-1, programmed death 1.