Figures & data
Figure 1. Clearance of P. aeruginosa from the acutely infected lung mouse model, 4h post challenge with live bacteria. Mice (Balb/c) were parenterally immunised with 10 µg antigen (KatA or OprF-I) or 106 colony forming units (cfu) heat killed Pa in Incomplete Freund’s adjuvant (as indicated) on days 0 and 14. Acute P. aeruginosa lung infection involved direct intra-tracheal (IT) inoculation of live P. aeruginosa (106cfu) into the lungs while briefly sedated with alfaxan (12 mg/kg) on day 21. Clearance was assessed at 4 h post live challenge. *P < 0.05 compared with non-immune.
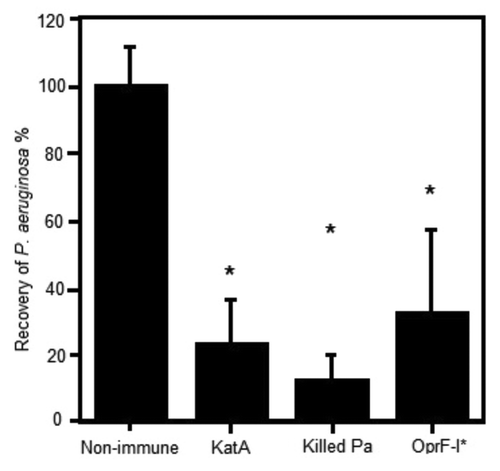
Figure 2. Clearance of live P. aeruginosa administered IT in agar. Mice were parenterally immunised as described in , except Kat A was mixed equally with Ad (10 µg each). Live challenge with P. aeruginosa via the trachea was administered on day 21 with 2x104 colony forming units in 0.5% agar. ⬜ Non-immune group (SEM = ± 44%); ⬤ KatA and Ad immunised group (SEM = ± 22%); ⬥ heat-killed P. aeruginosa immunised group (SEM = ± 12%). Data presented as the mean of n = 5–6 mice per group. S.E.Ms were too small to show graphically and are given above in the legend text.*P < 0.05 compared with the non-immune.
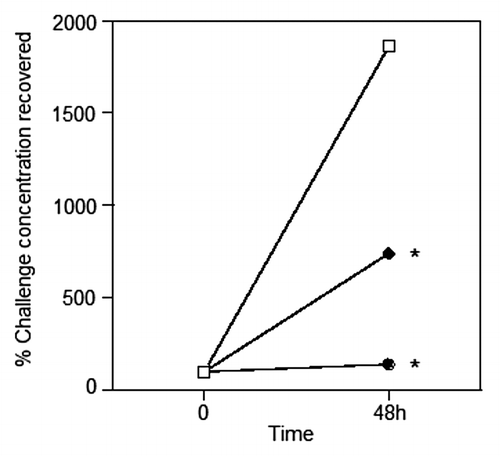
Figure 3. Vaccinating to clear an established chronic P. aeruginosa infection. This animal model was established in our laboratory to determine whether immunisation was effective in combating acute bacterial challenge in mice that had already been chronically infected with P. aeruginosa. (A) In this model, Balb/c mice received 104 colony forming units (cfu) of live P. aeruginosa in 0.5% agar intra-tracheally (IT) into the lungs on day 0. On day 7, they received a second dose of 104 cfu live P. aeruginosa in PBS (IT). One group of control non-immune mice⬜, received only agar on day 0 and PBS on Day 7, prior to acute live P. aeruginosa challenge at day 35, this provided a control group not previously exposed to an infection. The mice were then immunised sub-cutaneously on days 14 and 28 with either PBS (control mice) or vaccines formulated in IFA. On day 35, mice received an acute infection of 106cfu live P. aeruginosa (IT). The mice were killed at 4 h post this infection and bronchial lavage samples collected for testing. (B) This figure illustrates the clearance of P. aeruginosa in the bronchial lavage following acute bacterial IT challenge, 106cfu live P. aeruginosa or PBS, at day 35 in each of the groups. Non-immune mice received PBS (IT) and were sham immunised. All the filled symbol groups received P. aeruginosa in agar (IT) at day 0. ⬥Non-immunised control group (but did receive infections on days 0 and 7); ⬤KatA/Ad immunised group; ▲ heat-killed Pa. Kat A was mixed equally with Ad (10 µg each); and the heat killed P. aeruginosa (HKPa) group was immunised with heat-killed P. aeruginosa. Data presented as mean ± SEM of n = 5–6 mice per group.
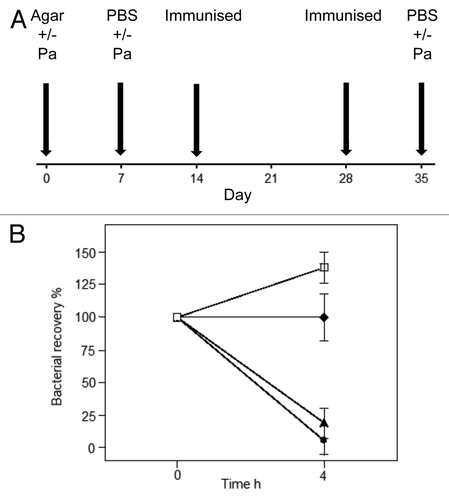
Figure 4. The histopathology of the lungs of Balb C mice immunised against Kat A/Ad, after acute exacerbation of chronic infection by P. aeruginosa. Immunised mice lungs showed reduced cellular recruitment, infiltration and lung consolidation and damage after the acute bacterial challenge compared with lungs from non-immunised, chronically infected control mice. (A) Shows the histological features of the lung in naïve mice, prior to chronic infection. Pulmonary bronchioles (PB) exhibit regular, intact epithelial surface and the epithelial walls of the alveolus (arrowed) encompass well defined expanded chambers, free of mucus. BV, blood vessel. (B) Illustrates the increased epithelial thickness (arrowed) and infiltration of nucleated cells adjacent to the alveoli resulting from establishment of chronic infection, within the mouse lungs prior to immunisation. (C) Illustrates similar characteristics resulting from the establishment of chronic infection in the lung in non-immunised mice, prior to bacterial challenge. As observed in (B), chronic infection has increased epithelial thickness (arrowed). (D) Shows the features of lung histology from chronically infected mice after immunisation against Kat A/Ad, prior to acute bacterial challenge. Although increased epithelial thickness is observed in the PB and there is evidence of localized cellular recruitment, the airways remain clear. (E) Demonstrates the damage induced in lung morphology from chronically infected mice in the absence of immunisation to Kat A/Ad, four hours after acute bacterial challenge. PB epithelia are thickened, alveolar walls and capillaries compromised leading to extravasation of blood into airways (#), increased cellular recruitment and consolidation of the lung through collapse of alveolar structure. (F) Demonstrates, in comparison to (E), that 4 h post bacterial challenge, the lung morphology of mice immunised against Kat A/Ad does not exhibit the epithelial damage, alveolar destruction, cellular recruitment or leakage of blood into the airways seen in non-immunised animals. The airways remain relatively clear, with minimal epithelial thickening (arrow). There is some evidence of increased mucus secretion (*) in smaller airways that have retained the mucus during histological processing of the lung. (G and H) Illustrates the lung histology, 24 h post bacterial challenge in non-immunised (G) and animals immunised against Kat A/Ad. In both groups, clearance of the acute damage observed in (E and F), has occurred, however the epithelial thickening, cellular infiltration and alveolar damage is more extensive in the lungs from non-immunised mice. Immunised mice still retain mucus within some of the airways however the extent of airway damage and consolidation appears less in this group.
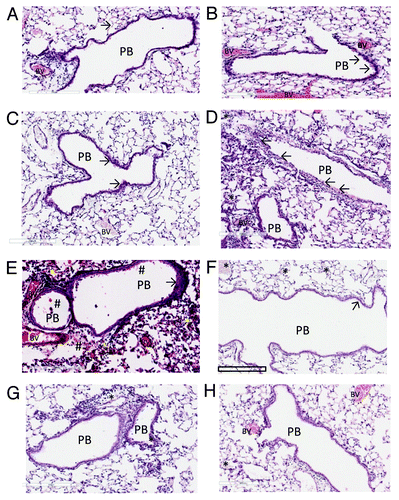
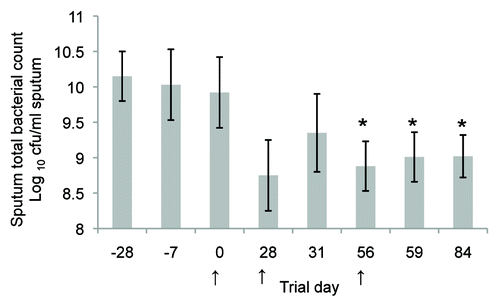
Figure 5. Clinical trial results showing total sputum bacterial counts (calculated as the log10 colony forming units /mL sputum, mean ± SEM) for the nine patients with bronchiectasis from a pilot open label study. The mean sputum bacterial count is significantly lower (P < 0.05; unpaired t test) for days 56–84 compared with the pre-screening day (Day 28) and initial trial culture (Day 7). Oral immunisation commenced on Day 0, Day 28, or Day 56 as indicated by arrows and continued for a 3 d period. Each subject received 2 × 1011 formaldehyde killed P. aeruginosa per day as an enteric coated capsule. All subjects were fasting for at least 6 h prior to the administration of the capsule.Citation59 Reproduced with permission of the University of Sydney from: RL Clancy, G Pang, Dunkley M, and AW Cripps Control of bacterial colonisation of the respiratory tract mucosa in man. In: Mucosal Solutions: Advances in mucosal immunology.Volume 1. Editors: Alan J Husband, Kenneth W Beagley, Robert L Clancy, Andrew M Collins, Allan W Cripps and David L Emery. University of Sydney, Sydney, Australia. pp 261–268. 1997