Figures & data
Figure 1. HPV 16 E5 peptide production. Immunoblotting of purified bacterial extracts was performed as in Material and Methods. Lanes 5 to 1–0.5 dilution of purified bacterial extracts; Lane M–6xHIS Protein Ladder (Qiagen).
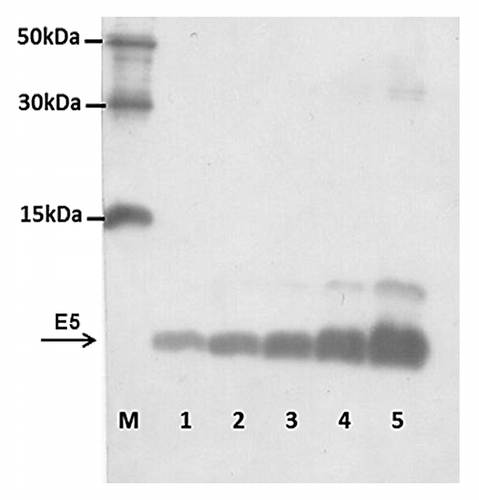
Figure 2. mRNA expression of the E5 gene in C3 cell line. Total cellular RNA was extracted from C3 cell line. cDNAs were synthesized by RT-PCR and specific amplified products were obtained with specific primers as described in “Materials and Methods”. Lane 1, E5 gene amplified from C3 RNA; Lane 2, E5 gene amplified from pCIE5 plasmid, as positive control; Lane 3, total C3 RNA without reverse transcriptase, as negative control; M, molecular weight marker VIII (Roche).
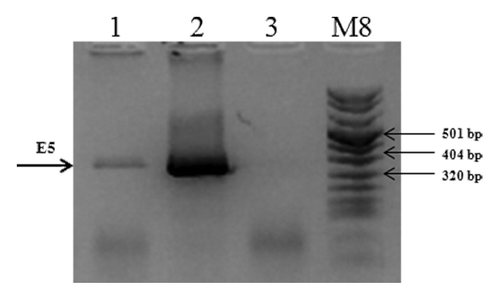
Figure 3. HPV16 E5 and E5Multi genes. The schematic representations of the E5 HPV16 gene (252 bp) with the position of CTL epitope sequences (cassettes A and B) and of the synthetic E5Multi gene (224 bp) with the position of the duplicated CTL epitope sequences (cassettes A and B) are shown together with the restriction sites for directional cloning. The aminoacid sequences of both epitopes are also indicated.
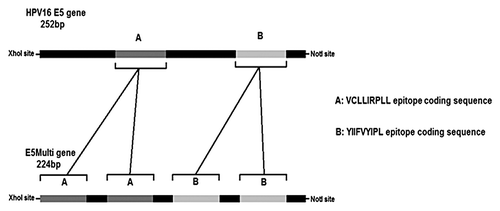
Figure 4. Prime/boost schedule for preventive or therapeutic immunizations. Five mice per group were used in both immunization schedules. (A) Preventive immunization schedule; the arrowhead (▲) indicates the day of blood sampling for ELISA test. (B) Therapeutic immunization schedule.
Figure 5. Preventive immunization with E5-based vaccines. (A) ELISA assay. Sera were collected from mice on day 14 after last boost and analyzed by E5 specific ELISA as described in “Materials and Methods”. Data are expressed as means for each group of mice ± SD (B) Cancer growth. Tumor development was assessed by palpation three times per week after C3 cell challenge. (C) Tumor burden. Five weeks after challenge mice were sacrificed and excised tumors weighted. Data are represented as mean of all of the mice in the groups ± SD.
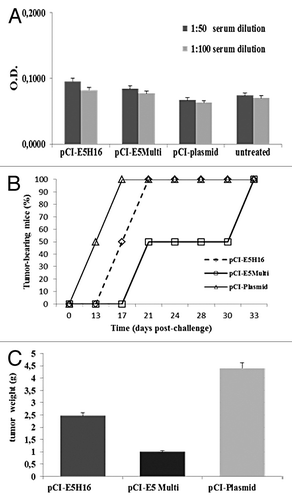
Figure 6. Cell-mediated immune responses in vaccinated mice. C57BL/6 mice were immunized by intramuscular administration of the various genetic vaccines as described in “Materials and Methods”. IFN-gamma production was measured in an ELISPOT assay after specific antigenic stimulation with E5 protein. Data are presented as fold-increase responses to the E5 protein, compared with mice vaccinated with empty vector, and they represent the means of all of the mice in the groups ± SD.
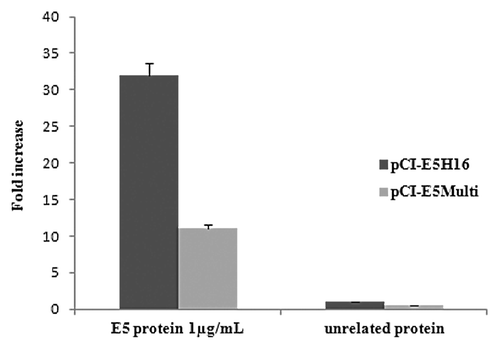
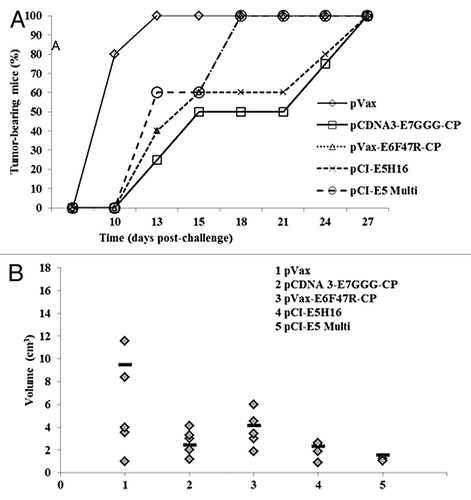
Figure 7. Tumor growth after therapeutic E5, E6 and E7 immunization. Fifty micrograms of plasmid DNA were administered by electroporation on days 3 and 10 with either the indicated vaccine preparations or saline solution as control. (A) Cancer growth. Tumor development was assessed by palpation three times per week. (B) Tumor burden. Tumor volume was measured 4 wk p.c. and calculated as in “Material and Methods”. Bars (▬) represent the mean value per each mouse group.