Figures & data
Figure 1 Participant disposition and reason for withdrawal at the end of the treatment phase (day 56) and at month 6, 7, 12, 24, 36, 48 and 60 for participants treated with a single 3.8 log10 dose of JE-CV and placebo with or without a booster dose of 3.8 log10 JE-CV at month 6. Treatment period withdrawals (to day 56): Group A: One Treated related adverse event (TRAE) (mild glomerulonephritis and anemia). Two military deployments/training. All three participants received JE-CV but not placebo. Group B: Two instances of protocol non-compliance. One participant with a protocol deviation received both JE-CV and placebo while one participant withdrawn for a reason in the “Other” category received only placebo. Second dose group withdrawals (to month 7): Five withdrawals—lost to follow-up. All participants received vaccine. Long term follow-up withdrawals: (1) One participant was withdrawn due to mouse brain derived, inactivated JE immunization administered between month 48 and month 60. This was required because the participant had no detectable JEV antibodies and was required to deploy to a JE endemic area. (2) A total of 125 participants did not attend the annual follow-up visit over 5 years, principally due to military deployments, leaving the service or posting outside the visitation range of the single center study site.
Figure 2 Kaplan-Meier survival analysis showing seroprotection (PRNT50 > 10) over time for 1-dose (Group C) and 2-dose (Group D) JE-CV groups from month 6 to 60. Kaplan-Meier survival analysis was used to determine the proportion of participants maintaining seroconversion to JE-CV from month 6 to 60 with separate analyses undertaken for participants given a single dose of vaccine and those given two doses of vaccine (log-rank test). Participant numbers “at risk”, “failed” and “censored” reflect those who attended each visit and a particular study population (i.e. safety or ITT populations) (). Month 7 was not performed for the single dose participant with a serology value at month 7. PRNT50 values reported at < 10 were converted to a PRNT50 = 5. If a PRNT50 value was missing for a visit and the participant returned for the next visit and was seroprotected at that time; the participant was assumed to have remained seroprotected at the time of the missing visit. If a PRNT50 value was missing for a visit and the participant returned for the next visit and was not seroprotected (i.e. PRNT50 < 10) at that time, the participant was assumed to have not been seroprotected at the time of the missing visit. For participants who missed two consecutive visits, only the data up to the missing visits was used for the Kaplan-Meier estimate.
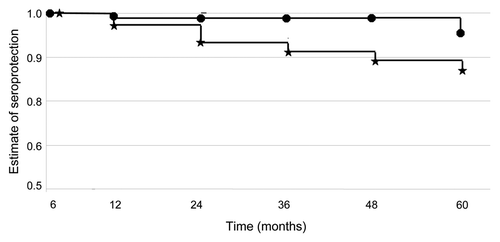
Figure 3 GMT and GMT ratio for 1-dose (Group C) and 2-dose (Group D) from month 6 to 60. The geometric mean ratio was calculated by ANOVA and was considered significant if the 95% CI did not contain the value one. Significant differences (p < 0.05) in the anti-JE-CV GMT from month 12 through month 48 are indicated by an asterisks.
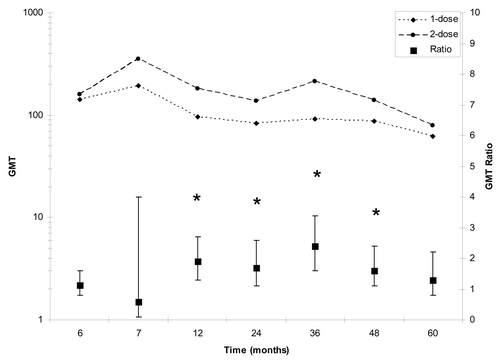
Table 1 Treatment related adverse events occurring in ≥1% of healthy participants following treatment with a single dose of JE-CV or placebo at Month 0, and following a booster dose of JE-CV at month 6 (safety population)
Table 2 Seroprotection and seroconversion to vaccine (JE-CV) from day 28 to month 60 in healthy participants (ITT population) treated with a single dose of JE-CV and placebo followed or not by a booster dose of JE-CV at month 6
Table 3 Kaplan-Meier estimate for persistence of neutralizing antibodies (PRNT50 ≥10) to vaccine (JE-CV) from month 6 to 60 in healthy volunteers (ITT population) treated with a single dose of JE-CV and placebo followed or not by a booster dose of JE-CV at month 6