Figures & data
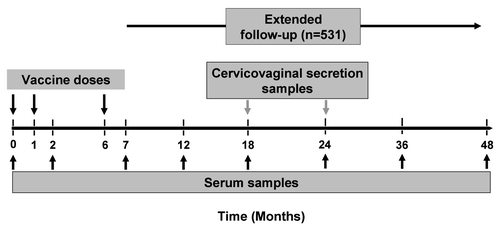
Figure 1 Subject disposition. n, number of subjects; 15–25 years, subjects 15–25 years of age at the time of first vaccine dose; 26–45 years, subjects 26–45 years of age at the time of first vaccine dose; 46–55 years, subjects 46–55 years of age at the time of first vaccine dose.
Figure 2 Antibody levels in women between 15 and 55 years of age initially seronegative for HPV-16 or HPV-18 antibodies (ATP immunogenicity cohort). GMT, geometric mean titer; Error bars, 95% confidence interval; 15–25 years, subjects 15–25 years of age at the time of first vaccine dose; 26–45 years, subjects 26–45 years of age at the time of first vaccine dose; 46–55 years, subjects 46–55 years of age at the time of first vaccine dose; Natural infection: GMTs of subjects from Study HPV-008 who were (a) HPV-16 or (b) HPV-18 DNA-negative and seropositive at baseline (i.e., who had cleared a natural infection. GMTs were (a) 29.8 EL.U/ml and (b) 22.7 EL.U/ml11; Plateau phase: GMTs of subjects from Study HPV-007 in women aged 15–25 years at Months 45–50 after the first vaccine dose (Total cohort). GMTs were (a) 397.8 EL.U/ml and (b) 297.3 EL.U/ml.Citation9
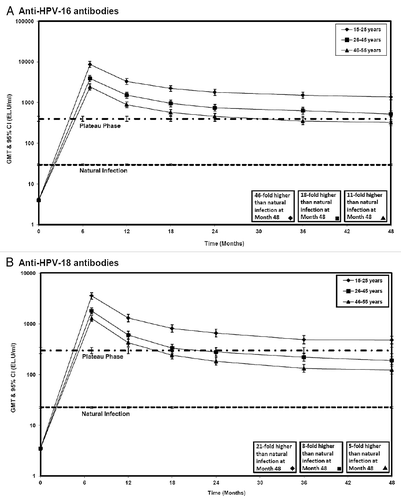
Table 1 Number of women reporting medically significant adverse events, serious adverse events, fatal serious adverse events or new onset of chronic diseases [classified by Medical Dictionary for Regulatory Activities (MedDRA) Primary System Organ Class and Preferred Term] and number of pregnancies and pregnancy outcomes over the 48 months of follow-up (total vaccinated cohort)