Figures & data
Figure 1. Architecture of JAK-STAT pathway components. (A) The constituent domains of JAK-STAT pathway components and their functionality. The PTK, SH2 and PTP domains contribute to the write, read and erase functionalities of the JAK-STAT pathway, as indicated, while the others have accessory roles. (B) The JAK-STAT pathway components and their domain assemblage.
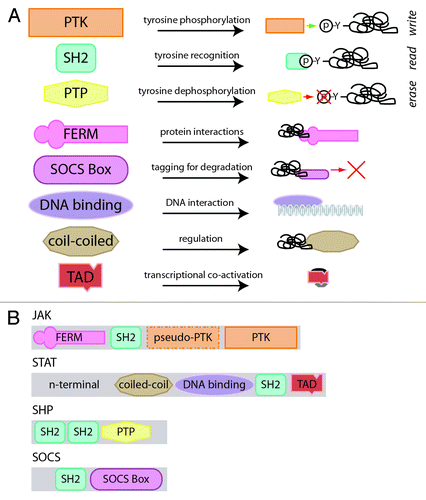
Figure 2. The canonical JAK-STAT pathway. (A) Ligand binding to a cytokine receptor results in dimerization and conformational changes that causes activation of JAKs associated with the Box 1 and Box 2 domains of the receptor intracellular domain. (B)The activated JAK is able to phosphorylate tyrosines on the receptor complex to create docking sites for signaling proteins, such as STATs. These are in turn phosphorylated, and then dimerize and translocate to the nucleus, where they bind to specific sequences in the promoters of responsive genes. (C) The pathway is extinguished by pre-existing SHPs, which serve to dephosphorylate tyrosine residues, or induced SOCSs that can act via several mechanisms, including JAK inhibition, steric interference at STAT docking sites, or mediating degradation of receptor signaling components.
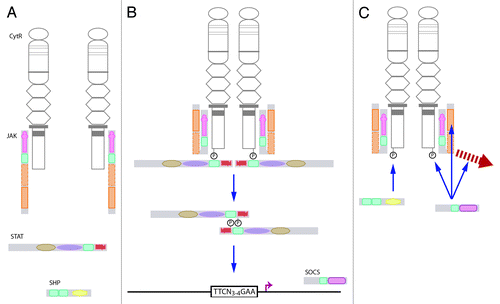
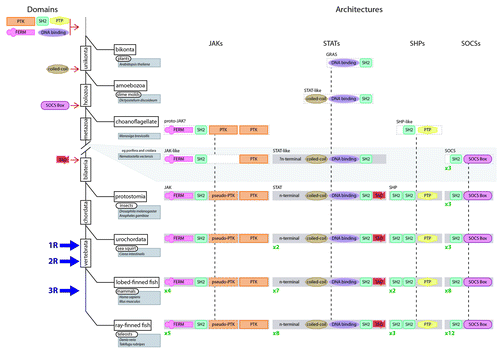
Figure 3. Assembly of the JAK-STAT pathway components during evolution. An abbreviated tree of life for the evolution of eukaryotes (solid black lines) including major divergence points (rectangles), timing of WGD events (1R, 2R and 3R) and key organisms (rounded rectangles)/species (shaded rectangles) covered by this review. The likely time of derivation of the constituent domains is shown to the left of the tree. The proposed evolutionary steps that formed the various JAK-STAT component architectures are shown to the right of the tree, including the subsequent reiteration of individual components, the number of which is shown in green.