Figures & data
Figure 1. JAK-STAT canonical signaling pathway in CNS. Hormones, growth factors and cytokines can induce JAK phosphorylation and activation. Activated JAKs phosphorylate STATs which in turn homo or heterodimerize. STAT dimers are then translocated to the nucleus where they bind to DNA.
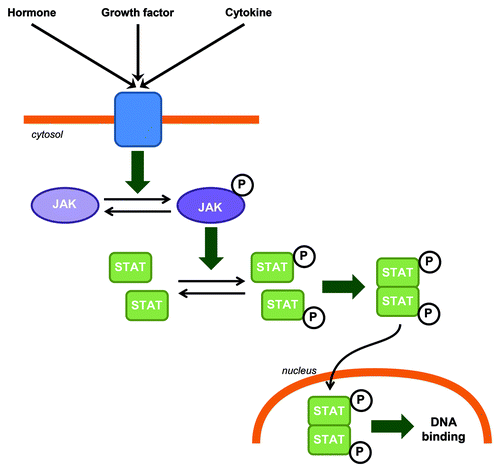
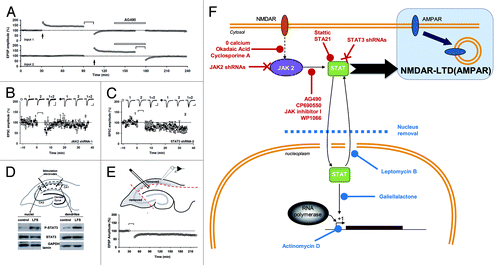
Figure 3. A non-nuclear role for JAK-STAT in synaptic plasticity (adapted from Nicolas et al.Citation2). (A) Pooled data of field recordings showing that a JAK inhibitor (AG490, 10 µM) can block the induction (but not the maintenance) of NMDAR-LTD. (B–C) Pooled data and representative currents of patch-clamp recordings (CA1 cells) in organotypic slices transfected with a JAK2 shRNA (B) and a STAT3 shRNA (C) showing that no LTD can be induced. (D) After stimulation, the area surrounding the stimulating electrodes (dendrites) and the CA1 cells bodies were microdissected. The nuclei were isolated from the cell bodies by centrifugation. The blots of P-STAT3 and STAT3 show that the phosphorylation of STAT3 was increased in both compartments after LTD. (E) Pooled data of field-recording performed on slices where the cell bodies have been removed, showing that the nuclei are not required for the induction of NMDAR-LTD. (F) Schematic summary of the data: Activation of NMDARs during synaptic stimulation leads to JAK2 phosphorylation and activation via a pathway involving Ca2+, protein phosphatase 2B (PP2B, inhibited by cyclosporine A) and Protein phosphatase 1 (PP1, inhibited by okadaic acid). JAK2 activates STAT3 which then translocates to the nucleus, but only the cytoplasmic actions of STAT3 are required for NMDAR-LTD. The 11 treatments in red are all able to inhibit LTD, whereas the four treatments in blue do not.