Figures & data
Figure 1. Model of the regulation of STAT signaling by PC1. During renal development, membrane-anchored, full-length PC1 may cause direct activation of STAT1 and STAT3 via JAK2 that is associated with its C-terminal cytoplasmic tail. Direct STAT1/3 activation by PC1 would be an intrinsic pathway that is independent of growth factors. It is currently unknown how the direct activation of STAT1/3 by full-length PC1 is regulated. It is possible that an—as yet unidentified—extracellular ligand may trigger STAT1/3 activation, or that the extracellular domain of PC1 engages in homotypic interactions. It is also possible that fluid flow may regulate this activity. During renal injury and in PKD, PC1 appears to undergo proteolytic cleavage that releases its cytoplasmic tail into the cytoplasm. This turns “off” the ability of the remaining membrane-anchored portion of PC1 to activate STAT1/3. However, the soluble PC1 tail can now translocate to the nucleus and co-activate STAT3 that has been activated by prior growth factor signaling. In addition to STAT3, the cleaved PC1 tail can also co-activate STAT6 (bottom) and STAT1 (not shown here). Therefore, the cleaved PC1 tail would have the ability to amplify different signaling pathways that lead to different cellular responses depending on the growth factor and cytokine environment.
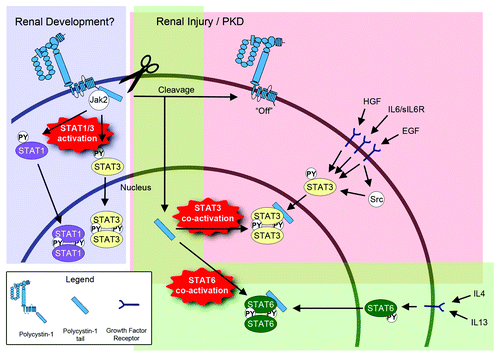
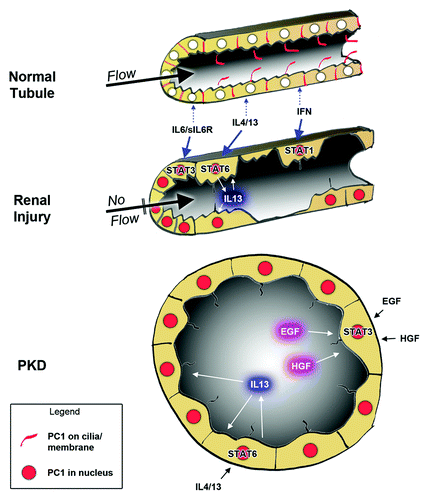
Figure 2. Model on how the cleaved, nuclear PC1 tail can lead to sensitization to STAT-activating growth factors. In normal renal tubule cells, PC1 expression is low and its localization to restricted to primary cilia and cell junctions (red). Renal injury leads to increased PC1 expression, cleavage of its cytoplasmic tail that then traffics to the nuclei (red) where it can co-activate STAT transcription factors. Depending on the growth factor/cytokine environment, different STATs will be activated. In injured tubules, this will lead to amplified STAT activity whereas intact tubules should be relatively insensitive (due to lack of nuclear PC1 tail). Different cytokines will lead to different responses. For example, IL13 secretion by M2 macrophages would cause STAT6 activation in tubule cells which may activate tissue regeneration. In contrast, interferon secretion by M1 macrophages may trigger cell death by activation of STAT1 in damaged tubule cells. In PKD, overexpression of (mutated) PC1 may lead to constitutively high levels of the nuclear PC1 tail which will hyper-sensitize tubule cells to cytokine signaling leading to inappropriate responses. Because factors secreted into cyst lumens cannot escape, positive feedback loops establish themselves which lead to persistent activation of STAT6 and STAT3.