Figures & data
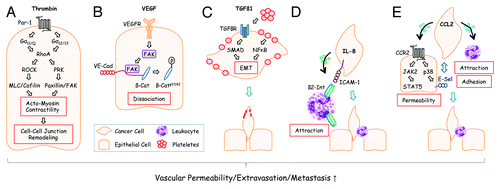
Figure 1. Disseminated tumor cells use different extravasation mechanisms. (A) Tumor cells can activate the coagulation system and generate thrombin from its precursor, prothrombin, at sites of metastasis.Citation23 Activation of the thrombin receptor PAR-1 on endothelial cells leads to G protein-mediated activation of RhoA and RhoA downstream signaling components that converge on the increase of actomyosin contractility and cell-cell junction remodeling. Adapted from reference Citation8. (B) VEGF, derived from tumor cells at the site of metastasis, activates focal adhesion kinase (FAK) in endothelial cells that subsequently binds to the cytoplasmic tail of VE-cadherin (VE-Cad). At this location, β-Catenin (β-Cat) is phosphorylated by FAK and dissociates from VE-cadherin leading to endothelial cell junctional breakdown.Citation6 Additional modes how VEGF affects vascular permeability have also been described.Citation24 (C) Plateletes can support metastasis in several ways: Platelet-derived TGFβ1 activates SMADs in tumor cells. Simultaneously, NFκB is activated by platelet-tumor cell contacts. Both synergize to induce epithelial-mesenchymal transition (EMT) thereby promoting extravasation. Adapted from reference Citation4. (D) Human melanoma cells readily extravasate when co-injected with human neutrophils into recipient mice. This effect is mediated by melanoma-derived IL-8 which attracts neutrophils and induces expression of β2-Integrin (β2-Int). Neutrophils anchor tumors cells to endothelial cells via contacts of β2-Integrin to extracellular matrix compounds and the cell adhesion molecule ICAM-1.Citation3 (E) Tumor cell-derived CCL2 can activate corresponding CCR2 receptors on endothelial cells thereby inducing JAK2/STAT5 and p38MAPK signaling pathways. Both pathways synergize to increase vascular permeability. p38MAPK induces expression of E-Selectin (E-Sel) in endothelial cells which promotes attachment of tumor cells. Simultaneously, monocytes are attracted by CCL2 which support extravasation of tumor cells through walls of capillaries with reduced barrier function. Adapted from reference Citation9.