Figures & data
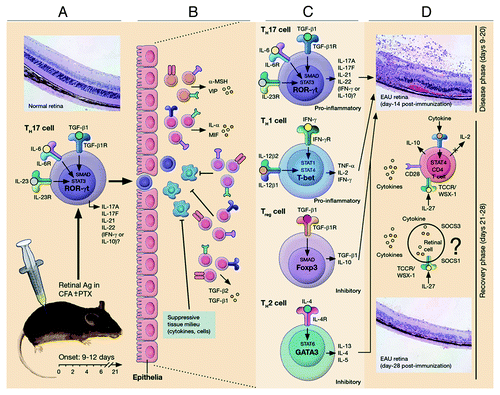
Figure 1. The IL-12 cytokine family and its effects on T-helper differentiation. The family is comprised of IL-12 (p35/p40), IL-23 (p19/p40), IL-27 (IL27p28/Ebi3) and IL-35 (p35/Ebi3), and each member interacts with high-affinity heterodimeric receptors comprising of the pairing between IL-12Rβ1, IL12Rβ2, IL-27Rα (WSX-1) or gp130. They mediate their biological effects through the activation of STAT pathways. The outcome of the response can be proinflammatory or immune suppression depending on the predominant IL-12 family cytokines secreted by dendritic cells during Ag priming.
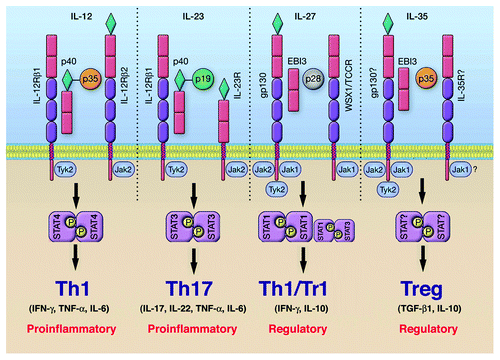
Figure 2. Cytokines and STAT pathways regulate T-helper cells that mediate uveitis and MS. Cytokine-binding facilitates recruitment and activation of requisite STAT proteins. Activated STATs induce the upregulation of lineage-specific master regulators such as T-bet, GATA3, ROR-γt or Foxp3 leading to the production of cytokines that promote the establishment and stability of the lineage. Cytokines produced by the T cell subset define the nature of the immune response. However, homeostatic balance of the various T-helper and regulatory T cells is orchestrated and exquisitely regulated by negative regulatory factors, including members of the SOCS family of latent cytoplasmic proteins.
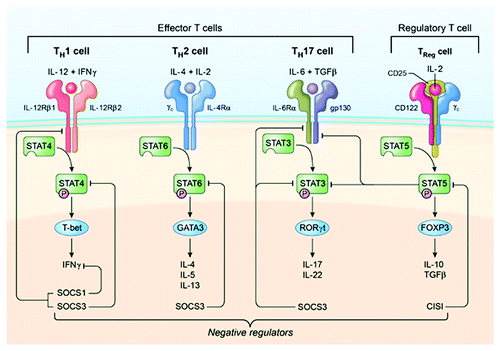
Figure 3. Schematic representation of early events leading to ocular inflammation, tissue destruction and induction of retinal protective mechanism in rodent model of uveitis. (A) Immunization of susceptible mouse strains (e.g., C57BL/6) in complete Freund’s adjuvant (CFA) triggers an immunological response characterized by abundance of Th17 and Th1 cells in lymph nodes, spleen and peripheral blood. (B) Activated Th17 cells expressing high levels of granzyme and activated α4β1 integrin facilitate breakdown of blood ocular barrier (BOB) retinal-blood extravasation into retina. The inflammatory cells entering the eye encounter hostile environment of the retina consisting of anti-inflammatory molecules (TGFβ, α-MSHa, VIP, MIF and IL-1rα) and resident retinal cells express inhibitory cell surface associated proteins (TGF-β, FAS/FAS ligand, CD46 and CD59). (C) Breakdown of BOB is accompanied by influx of other inflammatory cells and all major T-helper subsets are detectable in the reina during EAU. (D) Eventual elimination of cells from retina derives from endogenous adaptive mechanisms of ocular immune privilege. IFN-γ/STAT1-induced IL-27 production by resident ocular cells and cytokine-induced expression of SOCS1 and SOCS3 by retinal cells contribute to mitigation of uveitis.