Figures & data
Table 1 Values of Kd for human recombinant EGF, cetuximab and nimotuzumab in EGF receptors from human and green monkey placentas
Table 2 Relationship between receptor affinities and incidence of acneiform rash for anti-EGFR monoclonal antibodies
Table 3 EGFR expression across treatment arms for nimotuzumab + RT and RT arm
Table 4 EGFR expression across treatment arms for nimotuzumab + CT + RT and CT + RT arm
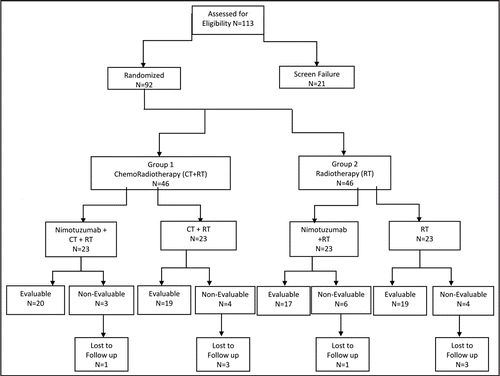
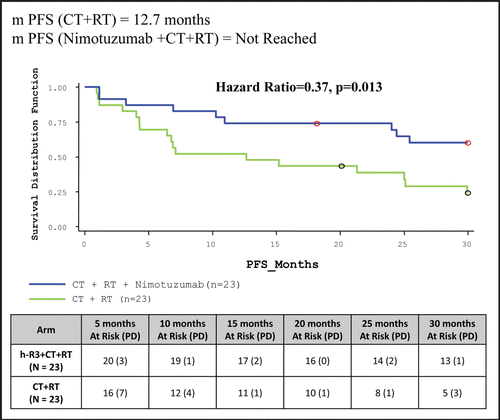
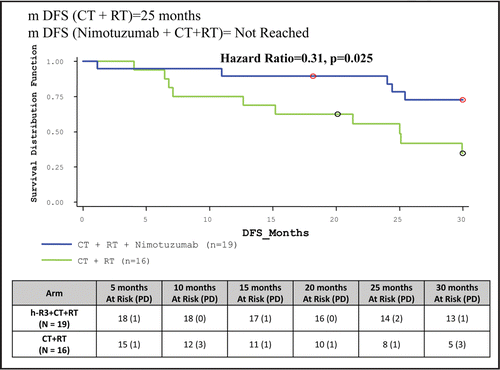
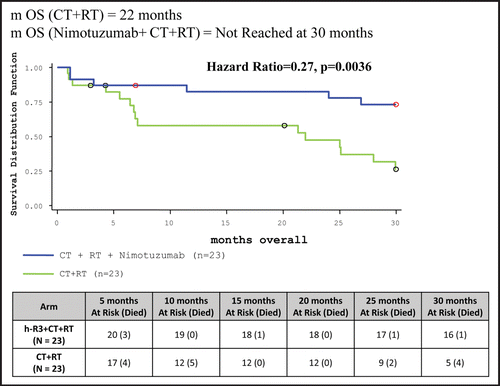
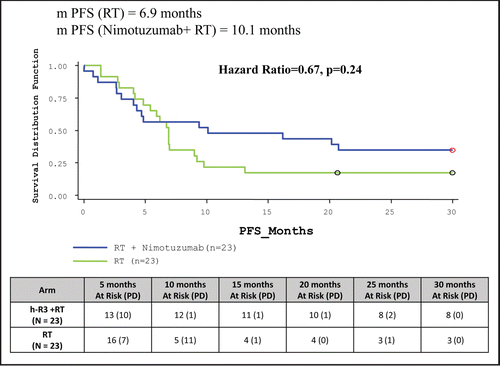
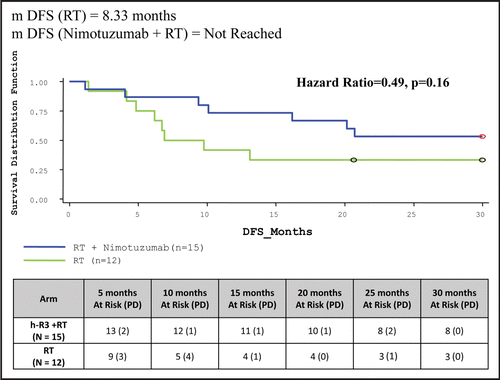
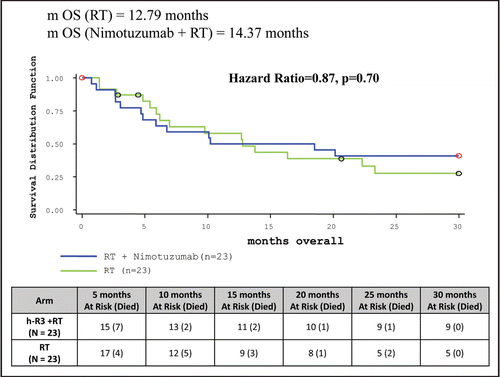
Table 5 Clinical study results: summary
Table 6 Completed and ongoing clinical trials with nimotuzumab