Figures & data
Figure 1 MDR status of AML cell lines as determined by rhodamine efflux. The AML cell lines, HL60, TF1-α and HEL9217, were incubated with rhodamine and efflux was evaluated by flow cytometry, as described in the Materials & Methods. HL60 cells are MDR- while TF1-α and HEL9217 cells are MDR+.
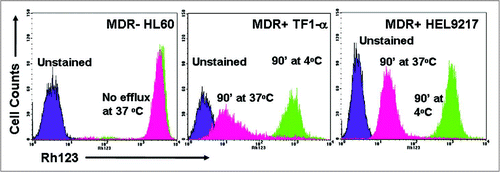
Figure 2 Lintuzumab (SGN-33) mediates signaling upon binding to CD33 and reduces cytokine and chemokine production. (A) HL60 cells and primary AML cells (#1 from a newly diagnosed patient and #2 from a patient with relapsed disease) were treated for 15 minutes (left) or various times (right) with 5 µg/ml lintuzumab or hIgG1κ. CD33 immunoprecipitates were prepared from cell lysates and analyzed by western blot analysis for tyrosine phosphorylation of CD33 and Shp-1 recruitment as indicated. Right panel represents a time course of Shp-1 recruitment to CD33 in lintuzumab-treated HL-60 cells. (B) KG-1 cells were treated with 2,500 ng/ml lintuzumab or 10,000 ng/ml hIgG1κ in the presence or absence 1 ng/ml TNF-α. Media were collected and assayed for cytokine or chemokine levels. Lintuzumab significantly reduced the production of IL-8, MCP-1 and IP-10. (C) Lintuzumab significantly reduced the production of IL-8 in a dose-dependent manner. Reduction of chemokine production was dependent upon binding to CD33 since the lintuzumab F(ab)2 fragment (1,000 ng/ml) was also effective. Data shown represent mean ± SD of duplicates from three separate experiments. **p < 0.01, and ***p < 0.0001 compared to hIgG1κ-treated cells by one-way ANOVA.
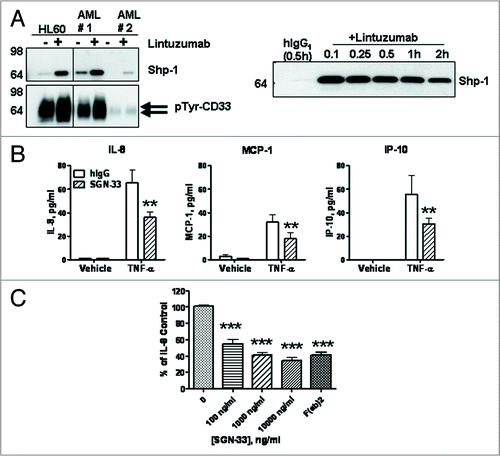
Figure 3 Lintuzumab (SGN-33) mediates effector functions. (A) ADCC activity with lintuzumab was evaluated in three AML cell lines and with cells from an AML patient with refractory disease using the standard 51Cr-release assay and NK cells isolated from FcγRIIIA 158V/V donors. (B) ADCP activity with lintuzumab was evaluated in 3 AML cell lines with primary human macrophages prepared from PBMCs. Phagocytosis (specific uptake of antibody-coated tumor cells by macrophages) was visualized by flow cytometry and by immunofluorescence (white arrows show uptake). (C) CDC activity with lintuzumab in U937 cells was measured with rabbit complement or human serum in the presence or absence of CRP blocking antibodies. Lintuzumab mediated CDC activity with rabbit complement or with human serum + CRP blocking antibodies. Data shown represent mean ± SD of triplicate values from three separate experiments.
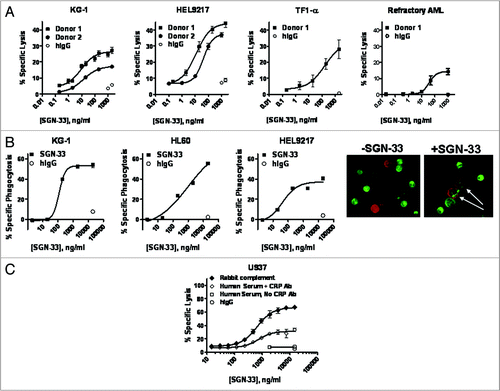
Figure 4 AML model development in SCID mice. Mice (n = 8 to 10 mice/group) were injected with HL60, TF1-α or HEL9217 AML cell lines and euthanized upon appearance of physical signs of disease development. Data shown are for HL60 model but similar results were found with the other models. (A) Expression of human CD33 in cells isolated from a solid tumor mass was maintained in culture when analyzed by flow cytometry after 6 days. The levels of CD33 in cells from the tumor masses were similar to those in the original tumor cell line. (B) Tissues were harvested from mice and the presence of human tumor cells (MHC-I+ CD33+) assessed by flow cytometry. Human CD33+ tumor cells were found in the blood, bone marrow, lymph nodes, and in solid tumor masses. (C) Human CD33 expression was detected by immunohistochemistry in a solid tumor mass isolated from bone.
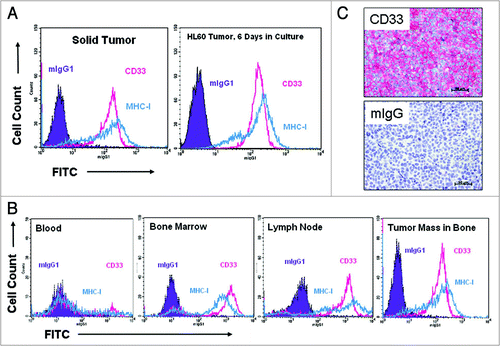
Figure 5 Efficacy of lintuzumab (SGN-33) in MDR− and MDR+ disseminated models of AML. Data shown are representative of findings in other studies. (A) In the MDR− HL60 model, mice (n = 8 to 10/group) were injected with 7.5 million cells and dosed one day or three days later with lintuzumab or hIgG1 (q4d × 4, arrows) or Mylotarg® (3 mg/kg, q7d × 2). Lintuzumab significantly enhanced survival of mice (p < 0.0001 to hIgG1-treated or untreated mice by log-rank test). (B) Plasma was collected from HL60 tumor-bearing mice three weeks after the last dose of lintuzumab and analyzed for cytokine levels. Data shown represent the individual and mean values obtained from 2 to 5 mice per treatment group. Lintuzumab appeared to significantly decreased the levels of IL-6 and IL-8 compared to untreated or hIgG1-treated mice. (C) In the MDR+ TF1-α model, mice were injected with 5 million cells and dosed 1 day or 3 days later with hIgG1, lintuzumab or Mylotarg®. Lintuzumab significantly enhanced survival of mice (p = 0.0002 to untreated or hIgG1-treated mice and p = 0.015 to Mylotarg®). (D) In the MDR+ HEL9217 model, mice were injected with five million cells and dosed 1 day or 3 days later with lintuzumab, hIgG1 or Mylotarg®. Lintuzumab significantly enhanced survival of mice compared to other treatments (p = 0.002 to hIgG1-treated group and p = 0.01 to Mylotarg®-treated or untreated mice).
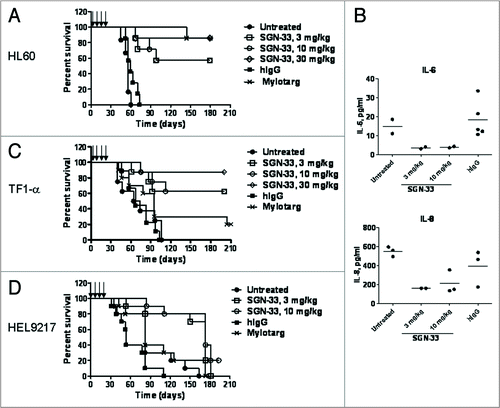
Table 1 Characterization of AML cell lines