Figures & data
Figure 1 SDS PAGE analysis of 2 of the ion exchange purified anti-TS1 mutants and the purified wild type anti-TS1 scFv used as standard in the quantitative ELISA. Lane 1: anti-TS1 scFv wild type 28 kDa (purified for the quantitative ELISA). Lane 2: anti-TS1 scFv mutant (low concentration approximately 10 nM). Lane 3: anti-TS1 scFv mutant (high concentration approximately 100 nM).
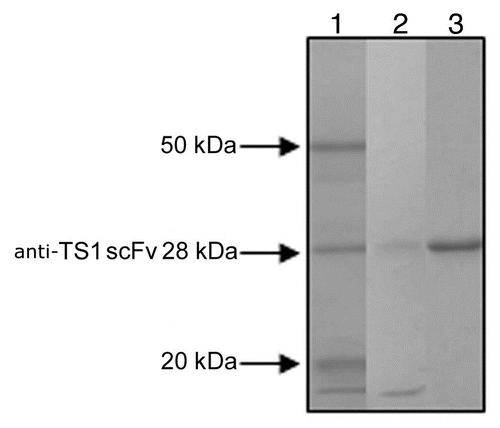
Figure 2 A representative kinetic plot, Ru signal versus sec, for one anti-TS1 scFv mutant (T97) at different concentrations (1,068 nM, 356 nM, 119 nM, 40 nM and 13 nM) by BIAcore as described (Langmuir model with local Rmax was used).
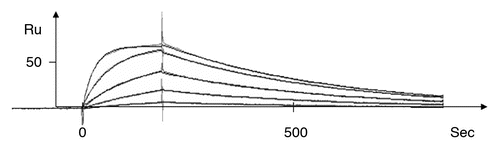
Figure 3 A plot of the association rate and dissociation rate constants of the 17 mutants. The wild-type anti-TS1 scFv and the anti-TS1 IgG demonstrating that all mutants display a higher dissociation rate constant (60–1,300) and 15 of the mutants a higher association rate constant (1.3–56) than the wild type scFv. Above and to the right in the figure, the affinity constant (KA) is presented by numbers and displayed as diagonal lines in the plot. The affinity for the mutants was reduced between 1.6–12,200 times.
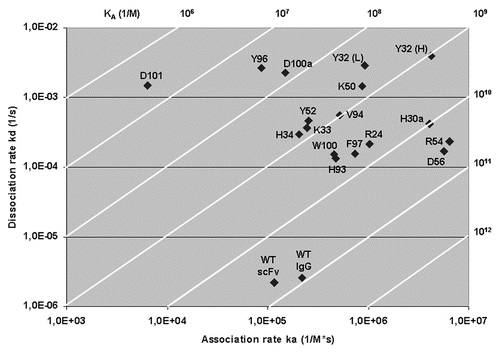
Figure 4 A three-dimensional model of anti-TS1 viewing the complementary determining regions (CDR) and antigen binding surface from the front. The upper part of the model represents the CDR of the heavy chain and the lower part the light chain. Grey color represents hydrophobic areas, yellow color represents polar parts, blue represent positive charges and red represent negative charges. Accessible residues are depicted with circles. Amino acids within thick black circles resulted in a substantial reduction (>2.5 kcal/mol) in binding energy (2.6–3.9 kcal/mol). Amino acids within thin black circles resulted in a moderate reduction in binding energy (1.6–2.3 kcal/mol). Amino acids with dashed circles resulted in a low reduction in binding energy (0.2–0.3 kcal/mol). One letter abbreviation of amino acids is used.
Table 1 For each amino acid residue position selected for mutagenesis, the table presents the approximate relative accessibility, the reduction in binding energy, the affinity reduction and the antigen binding frequency for antibodies that bind protein antigensCitation22