Figures & data
Figure 1 Enrichment of naïve B cells from human tonsil tissue. Mononuclear cells (MC) prepared from a Ficoll-Hypaque gradient were enriched for B cells using negative magnetic separation. Samples of the mononuclear cells (A–C) and the enriched B-cells (D–F) were phenotyped by gating for CD45 positive cells and staining for CD19 and CD20 (A and D) and also by staining for surface IgD, IgM and/or IgG expression (B, C, E and F). Results from a representative sample are shown.
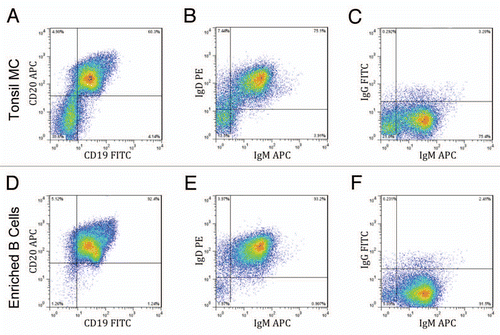
Figure 2 (A) Representative EliSpots displaying IgG secretion. (i) Freshly isolated naïve B cells from human tonsil [mean = 0.33 cells (0.0% positive)]. (ii) Total mononuclear cell population [mean = 17 cells (0.23% positive)]. (iii) B-cell population after undergoing Immunologix platform technology [mean = 438 cells (14.6% positive)]. All samples plated at 3 × 103 cells/well and done in triplicates. (B) Profile of IgG subclasses expressed by B-cell libraries. Duplicate experiment with two libraries.
![Figure 2 (A) Representative EliSpots displaying IgG secretion. (i) Freshly isolated naïve B cells from human tonsil [mean = 0.33 cells (0.0% positive)]. (ii) Total mononuclear cell population [mean = 17 cells (0.23% positive)]. (iii) B-cell population after undergoing Immunologix platform technology [mean = 438 cells (14.6% positive)]. All samples plated at 3 × 103 cells/well and done in triplicates. (B) Profile of IgG subclasses expressed by B-cell libraries. Duplicate experiment with two libraries.](/cms/asset/d37b05fa-d8e4-4c5d-a8ca-b1d715cdff17/kmab_a_10914774_f0002.gif)
Figure 3 Primary screening of human B-cell supernatants. After at least 12 days, supernatant from each well of the 96-well plates containing transformed human B cells was added to microtiter plates on which antigen was immobilized and captured human IgG detected using an HRP conjugated anti-IgG-Fc antibody. The data from the wells were sorted in descending order and expressed as a line graph. Supernatants from at least four B-cell libraries (48 plates total) are represented for each antigen. The inset indicates the percentage of supernatants that returned an optical density (OD) of greater than 1.0.
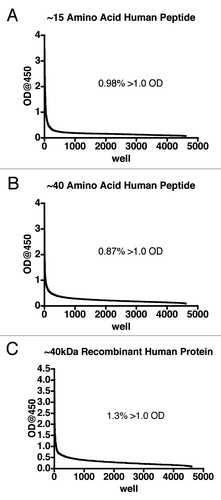
Table 1 Cellular yields from tonsil tissue
Table 2 B-cell transformation efficiency using spinfection with 10× concentrated EBV supernatant