Figures & data
Figure 1 European biosimilar guidelines. The EMA began with an overarching guideline on biosimilars and then general guidelines, before issuing product class specific data requirements. The EU Guidelines that have been finalized are indicated in blue. A draft guideline for mAbs is currently available for public comment.Citation25
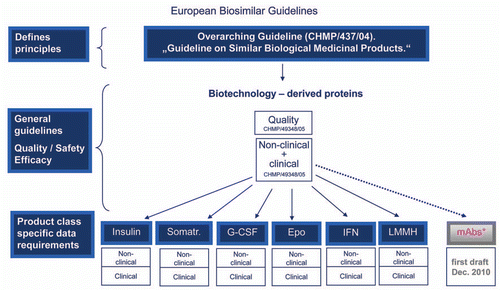
Figure 2 Analyzing complex product attributes over time. The bG2 glycan structure was quantified by Sandoz in many batches of commercial product distributed by the originator in the EU (light blue) and the US (dark blue). Expiry date of the product batches is listed on the x-axis and relative amount of product attribute enrichment is listed on the y-axis. Pre-shift quality refers to the content of the attribute prior to a manufacturing change and post-shift quality after the manufacturing change.
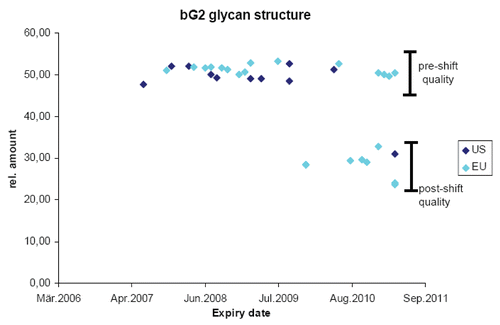
Figure 3 Biosimilar development process. The development of a biosimilar relies on creation of a design space based on analysis of the reference product and then iterative development of a biosimilar to fit the chosen specifications. There is no access to, nor need for, originator data at any point in this process. The early process development is essential, and later development cannot compensate for this initial generation of a “highly similar” candidate product. As the complexity of the reference product increases, the initial development becomes more challenging, and the likelihood that multiple iterations will be needed increases.
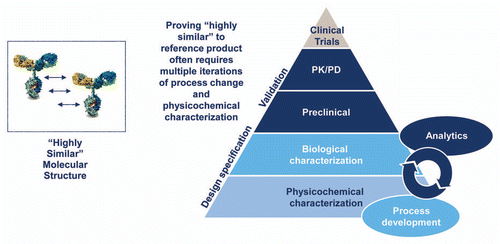
Figure 4 Biosimilarity goal posts. The “goal posts” of biosimilarity are established by the biosimilar sponsor by their analysis of the distribution of product attributes present in the reference product pre- and post-manufacturing change. They then use these to select the design space for their biosimilar candidate. While the complete quality range may be quite broad for the life time of the reference product, the biosimilar sponsor will select a tighter range of control for their biosimilar product.
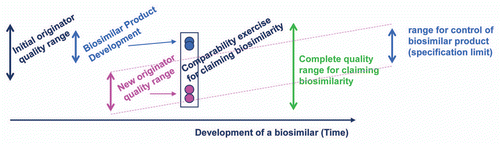
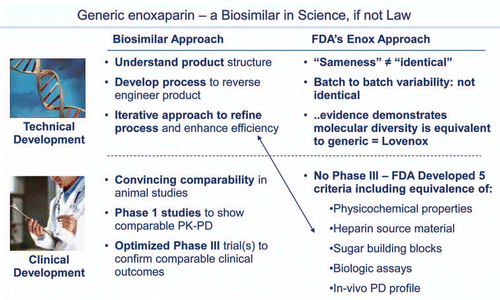
Figure 5 The one US marketed generic biologic: enoxaparin. Generic enoxaparin was approved by FDA on July 23, 2010. Although not a “biosimilar” insofar as it was not approved through the new biosimilar 351(k) regulatory pathway, scientifically it is a fully interchangeable generic biologic and many of the FDA considerations anticipated for biosimilars s apply.