Figures & data
Figure 1 Adeno-TWEAK or Fc-TWEAK treatment is efficacious in tumor xenograft model. (A) Efficacy of adeno-TWEAK treatment in MDA-MB-231 xenograft model is shown. MDA-MB231 cells were grown subcutaneously in nude mice and a single dose of adeno-TWEAK or adeno-GFP was administered intratumorally at day 9 post-implantation. Tumor volume is plotted over time, indicating marked reduction in tumor size of adeno-TWEAK treated mice. Statistical significance (p < 0.007) was achieved at each time point beginning at day 23 and continuing through the end of the study (day 76). (B) Actual tumor weights were determined upon dissection at termination of the study (day 76) as shown, indicating significantly reduced tumor mass in the adeno-TWEAK treated group compared with the adeno-GFP group. Shown are averages with standard deviation. Statistical significance (t-test) is shown. (C) H&E staining of tumors at termination of the study shows reduced tumor tissue in the adeno-TWEAK treated group compared with the adeno-GFP treated group. Ki-67 staining reveals reduced proliferation in tumors from the adeno-TWEAK treated mice compared with adeno-GFP treated group. TUNEL staining demonstrates increased levels of apoptotic cells in tumors from the adeno-TWEAK treated mice compared with the adeno-GFP treated group. (D) Efficacy of Fc-TWEAK treatment in MDA-MB-231 xenograft model is shown. Nude mice bearing MDA-MB-231 tumors were given biweekly intratumoral administration of Fc-TWEAK or vehicle control beginning at day 19 post-tumor cell implantation. Tumor volume is plotted over time, indicating a marked reduction in tumor size of Fc-TWEAK treated mice. Statistical significance was achieved (p < 0.02) at each time point beginning at day 54 and continuing through termination of the study (day 99). (E) Comparison of tumor weights as determined upon dissection at termination of the study (day 99) is shown, indicating significantly reduced weights in the Fc-TWEAK treated group. Shown are averages with standard deviation. Statistical significance (t-test) is shown.
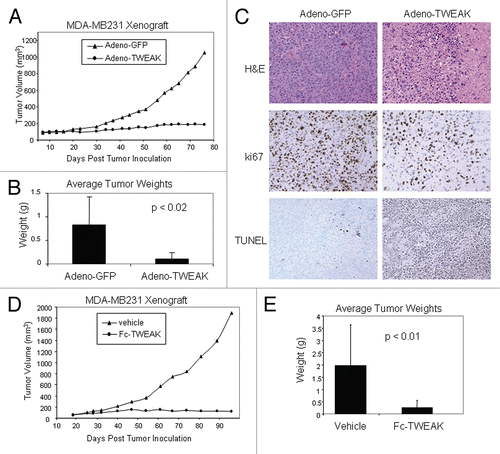
Figure 2 BIIB036 binds Fn14 and blocks TWEAK binding to Fn14. (A) Direct FACS binding assay of BIIB036 to 293E cells transiently transfected with full-length Fn14 from various species is shown. An empty vector (“control”) was included as a negative control in this experiment. BIIB036 exhibits similar binding to human, cynomolgous monkey, rat and mouse Fn14. The geometric mean fluorescence intensity (MFI) is plotted. (B) FACS competition assay of BIIB036 binding to Fn14-expressing WiDr cells in the presence of TWEAK is shown. WiDr cells were incubated with mFc-TWEAK in the presence of varying concentrations of BIIB036, human Ig control, or recombinant soluble TWEAK. Binding of mFc-TWEAK to the cells was detected with a labeled anti-mouse Ig antibody. mFc-TWEAK binding was inhibited at increasing concentrations of BIIB036 (or recombinant TWEAK). The geometric mean fluorescence intensity (MFI) is plotted.
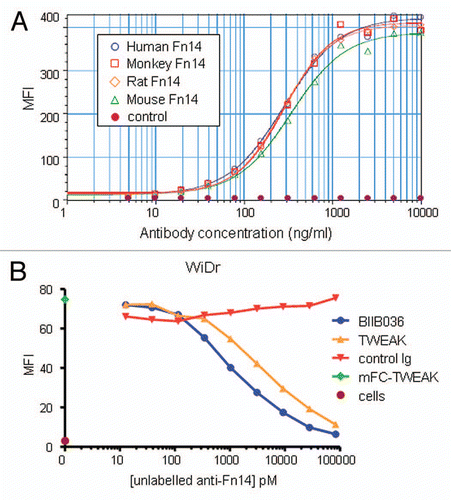
Figure 3 BIIB036 exhibits agonistic activity. (A) IL-8 release assay in A375 cells is shown. Supernatants from A375 cells incubated for 36 h with mBIIB036, but not control Ig, showed increased levels of IL-8, as measured by ELISA, in a dose-dependent fashion. Fc-TWEAK was included as a positive control in this experiment. (B) Protein extracts were prepared from WiDr and MDA-MB-231 cells incubated with BIIB036 (1 µg/ml) or Fc-TWEAK (100 ng/ml) for 24 h. Western blotting with an anti-NFκB-p52/p100 antibody detects p100 and the p52 cleavage product. p52 is apparent in BIIB036-treated WiDr and MDA-MB231 cells similar to the induction observed in response to Fc-TWEAK.
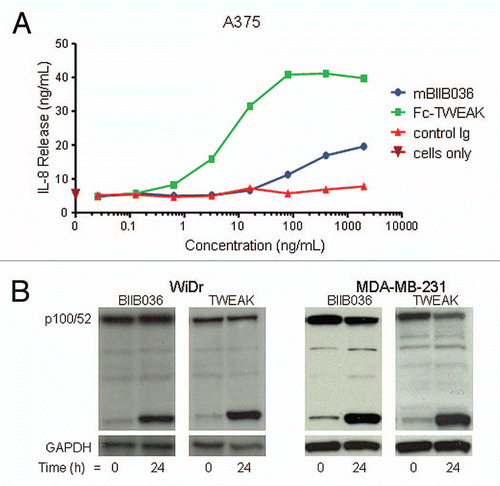
Figure 4 BIIB036 can induce tumor cell killing in vitro. (A) MTT assay was performed with WiDr cells treated with a range of doses of Fc-TWEAK, BIIB036 or human Ig control antibody in the presence of IFNγ for four days. Results are graphed as percent survival relative to control IFNγ treated WiDr cells. (B) A TUNEL assay was performed with WiDr cells treated with BIIB036 for 48 h in the presence of IFNγ. TUNEL-positive cells are observed in BIIB036-treated but not control cells. Fc-TWEAK was included in this experiment as a positive control. (C) MTT assay was performed with NCI-N87 cells treated with a range of doses of Fc-TWEAK, BIIB036-multimer, BIIB036 or Ig control in the presence of IFNγ for 3 days. Results are graphed as percent survival relative to control IFNγ treated NCI-N87 cells. (D) MTT assay was performed with MDA-MB-231 cells treated with a range of doses of Fc-TWEAK, BIIB036-multimer, BIIB036 or Ig control in the presence of IFNγ for four days. Results are graphed as percent survival relative to control IFNγ treated MDA-MB-231 cells.
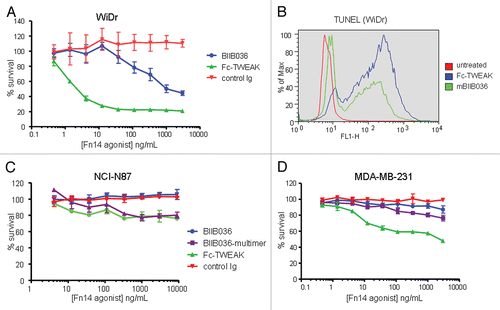
Figure 5 Multimerization of BIIB036 results in enhanced anti-tumor activity. MTT assay was performed with WiDr cells in the presence of IFNγ treated with a range of doses of BIIB036 or control Ig, in the presence or absence of a secondary cross-linking antibody, or with BIIB036-multimer, for 4 days. Results are graphed as percent survival relative to control IFNγ treated WiDr cells.
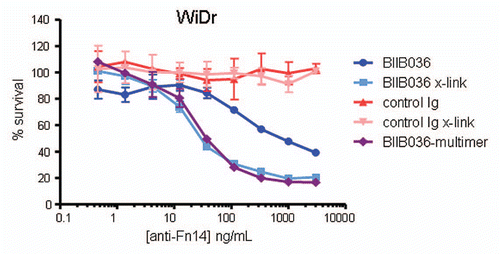
Figure 6 Cell killing in multiple tumor cell lines is TNF independent. MTT assays in (A) WiDr cells and (B) SKOV-3 cells were performed in the presence or absence of the anti-TNF inhibitor, Adalimumab (500 pM). Cell survival is plotted as a function of concentration of Fc-TWEAK, BIIB036-multimer or BIIB036. Only in SKOV-3 cells, but not in WiDr cells, is tumor cell killing inhibited in the presence of Adalimumab.
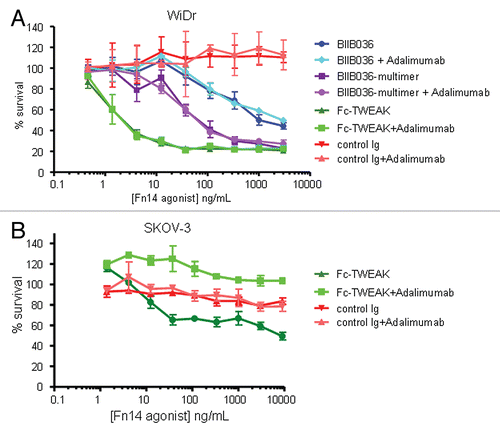
Figure 7 BIIB036 exhibits ADCC activity in vitro. ADCC assays were performed in (A) MDA-MB-231 and (B) WiDr cells with a 5:1 ratio of NK to target tumor cells in the presence of increasing concentrations of antibody. BIIB036 (hIgG1) was tested and compared to other Fc versions of the antibody (hIgG1agly or hIgG4Pagly) or control human Ig. Percent lysis is shown. Only BIIB036 exhibited activity in this assay.
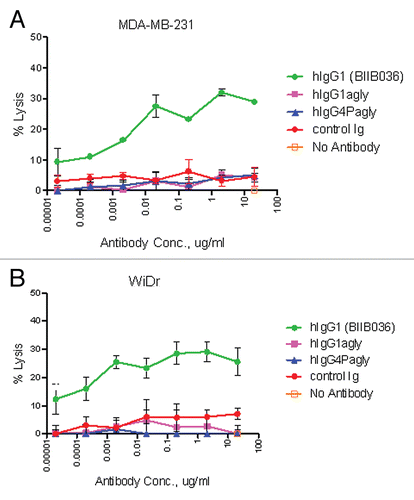
Figure 8 BIIB036 inhibits tumor growth and induces apoptosis in WiDr colon tumor xenograft model. (A) Efficacy of BIIB036 treatment in WiDr colon carcinoma xenograft model is shown. Tumor volume as a function of time in WiDr xenograft model is plotted. Nude mice implanted with WiDr tumor cells were treated with varying doses of BIIB036 (12.8, 6.4, 3.2, 1.8 or 0.9 mg/kg) or human Ig control (12.8 mg/kg) on a weekly basis for 6 weeks starting when the tumors were approximately 250 mm3. Data are mean ± SEM of 10 mice per group. Dose-responsive anti-tumor activity is observed, with significant efficacy evident at all doses relative to control group (p value < 0.001 on day 61 for dose groups 3.2 mg/kg and higher). (B) Percent change in body weight of each group of mice from the WiDr xenograft experiment in (A) is plotted as a function of time. No reduction in body weight was observed in any of the treatment groups. (C) WiDr tumors harvested from mice treated with mBIIB036 (6.4 mg/kg) were sectioned and stained for cleaved caspase-3. Increased cleaved caspase-3 staining is observed at 24 and 48 h post-BIIB036 treatment. (D) Graphical presentation of cleaved caspase-3 staining in WiDr tumors from mice at 1–7 days following treatment with mBIIB036 (6.4 mg/kg). The percent of nuclei staining positive for cleaved caspase-3 is plotted. Average values with standard deviation for n = 5 mice per group are shown.
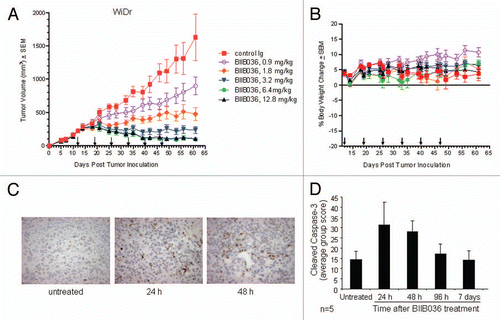
Figure 9 BIIB036 inhibits tumor growth in NCI-N87 gastric and MDA-MB-231 breast tumor xenograft models and reduces lymph node metastases. (A) Efficacy of BIIB036 treatment in NCI-N87 gastric xenograft model is shown. Nude mice implanted with NCI-N87 tumor cells were treated with varying doses of BIIB036 (3.2, 6.4 and 12.8 mg/kg) or human Ig control (12.8 mg/kg) on a weekly basis for 6 weeks starting when the tumors were approximately 250 mm3. Data are mean ± SEM of ten mice per group. Statistically significant robust anti-tumor activity is observed at all doses relative to control Ig over the entire dosing period (p < 0.0001). (B) Efficacy of BIIB036 treatment in MDA-MB-231 breast xenograft model is shown. Nude mice implanted with MDA-MB-231 tumor cells were treated with varying doses of BIIB036 (6.4, 12.8 and 25.6 mg/kg) or human Ig control (25.6 mg/kg) on a weekly basis for 6 weeks starting when the tumors were approximately 250 mm3. Robust anti-tumor activity compared to the control is observed at all doses (p value < 0.000001 for all BIIB036 groups relative to control group at day 58, one week following the final dose). For all dose groups, statistically significant anti-tumor efficacy was maintained through the entire dosing period and beyond (p < 0.0001 compared to control from days 16 to 70). (C) Metastatic tumor burden in the lymph nodes is shown. Each bar represents the mean combined volume of right and left axillary lymph nodes. A dramatic reduction in metastatic volume is observed in BIIB036-treated mice compared with mice treated with human Ig control (p < 0.03, t-test).
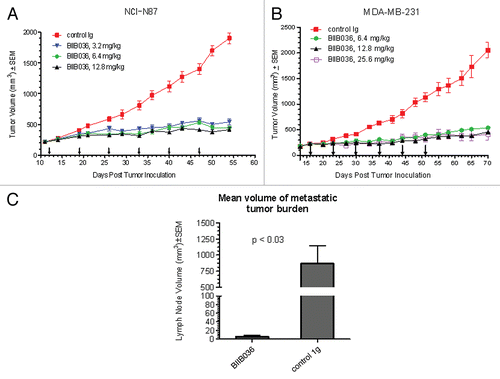
Figure 10 BIIB036 exhibits superior anti-tumor activity in vivo relative to versions of the antibody with reduced effector function. (A) MDA-MB-231 and (B) WiDr tumor xenograft model were used to compare the efficacy of different Fc versions of BIIB036. BIIB036 (hIgG1) exhibited superior efficacy compared with the other Fc versions of the antibody (hIgG1agly and hIgG4Pagly) when dosed weekly for a 6 week period. Control human Ig (dosed at 12.8 mg/kg in the WiDr study and 25.6 mg/kg in the MDA-MB-231 study) was included as a negative control.
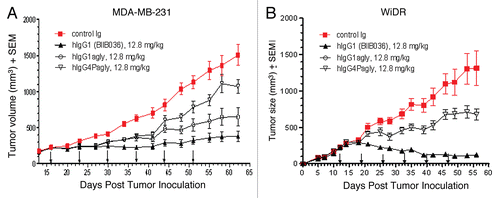
Table 1 Effect of BIIB036 on metastasis in MDA-MB-231 breast tumor model