Figures & data
Figure 1 Comparison of the isoforms of different lots of IgG1 product produced using Process 1 and 2. (A and J) Markers; (B) Process 1, Lot 1; (C) Process 1, Lot 2; (D) Process 2, Lot 1; (E) Process 2, Lot 2; (F) Lot 1, Location 1; (G) Lot 2, Location 2; (H) Lot 3, Location 1; (I) Lot 4, Location 2.
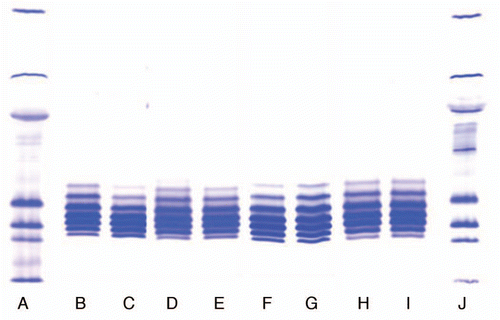
Figure 2 Comparison of isoforms of the IgG1 product prepared with Process 3 in two locations. (F) Marker; (G) Lot 1, Location 1; (H) Lot 2, Location 2; (I) Lot 3, Location 1; (J) Lot 4, Location 4.
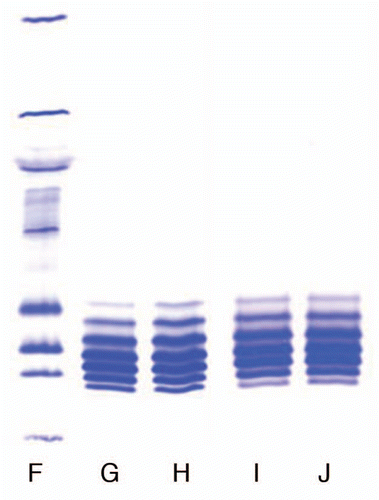
Figure 3 Negative-ion MALDI spectrum of 2-AA N-linked glycans released from IgG1 product RS by PNGase F. Sugar residues are GlcNAc (blue square), Fuc (yellow triangle), Man (green circle), Gal (pink circle) and NGNA (black diamond).
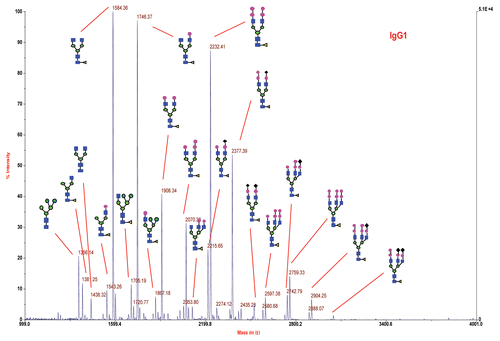
Figure 5 Effect of sialidase and carboxypeptidase treatment on the IgG1 product. The Isoforms 1 and 2 are not present in the sialidase and carboxy peptidase treated sample but they are present in sialidase treated sample. Isoforms 6, 7, 8 are not present in sialidase treated sample indicating that they contain sialic acid.
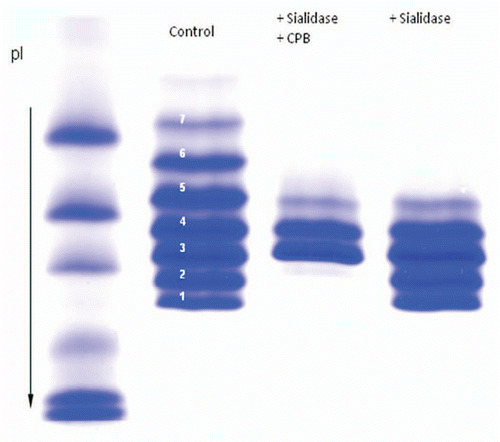
Figure 6 LC/MS/MS peptide map of IgG1 isoforms. The percent of Lysine of the isoforms 1–8 were determined to be 80.2, 54.3, 21.0, 14.3, 8.5, 5.4, 5.1 and 5.7, respectively indicating lysine truncation in isoforms 5–8.
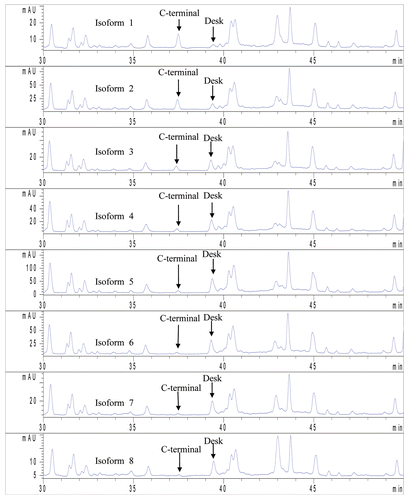
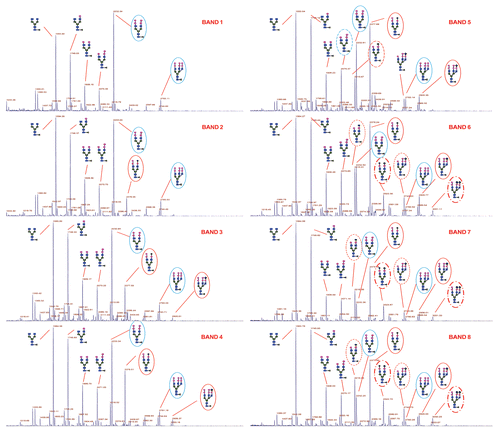
Table 1 Relative abundance of oligosaccharides verse G0F for each band
Table 2 Biological activity of the IgG1 product