Figures & data
Figure 1. Formation of miRs typically occurs when two complementary TEs inserted into opposing strands are then subsequently transcribed. (A) Cartoon representation illustrating the proposed origin of many miRs. A miR hairpin is depicted just above an arrow indicating read through transcription from a positive strand transposable element (TE) into an adjacent negative strand TE. Transcriptional read through would result in an imperfect RNA hairpin being produced which could potentially be recognized and processed by the RNAi machinery with each stem corresponding to the terminal nucleotides of the contributing TEs. (B) Pan troglodytes miR-95 alignment to the RepBase data set. All repetitive elements taken from RepBase (indicated by open rectangles) occurring within 200 bp (5′ and 3′) have been included in the scale diagram. The repetitive element annotationsCitation23 are described immediately beneath the diagram as “Element 1, Element 2, etc…” as they occur 5′ to 3′. “Base Positions” refers to base pair location in the genome occupied by the miR hairpin (according to the current Ensembl assembly; www.ensembl.org). All loci have been shown with respect to the positive strand and the orientation of internal repetitive elements illustrated by their relative position above (5′ to 3′) or below (3′ to 5′) the center line. Repetitive element base pair positions are relative to the distance (±) from the first nucleotide of the pre-miR (as occurring on the positive strand). Figure directly adapted from reference Citation24.
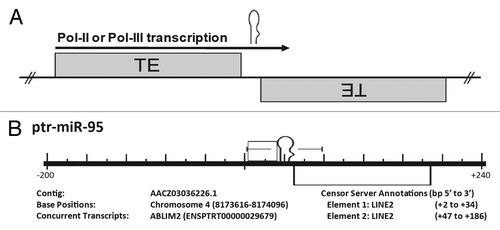
Figure 2. Cartoon illustration of the genomic events believed to be responsible for the formation of many miRs. Origin of numerous miRs occurs when random TE insertions gives rise to a beneficial regulatory adaptation within the organism. Subsequent transcription of this TE interface by RNA polymerase followed by RISC processing can lead to miR establishment if the resulting small RNA confers some advantage in gene expression.
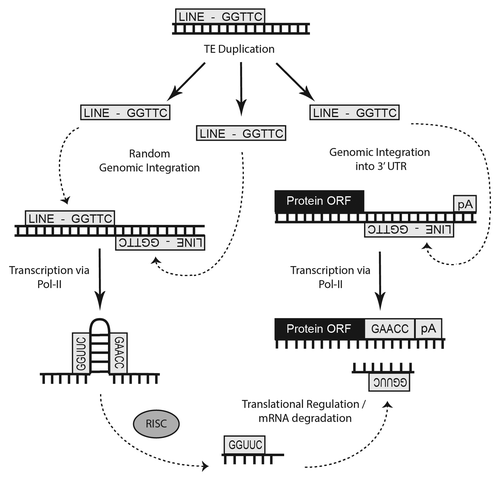
Table 1. Summary of miR loci transposable element origins
Figure 3. Alignment of microRNA-444 family. Alignment of the eight miR-444 hairpins is illustrated. Each individual hairpin sequence is shown with the associated species on the right and the miRBaseCitation28 identifier on the left. In all, six unique miR-444 hairpin origins were characterized by familial inclusion. *, indicates 100% conservation of the nucleotide. Grey shading indicates specific miR hairpins initially identified as bearing significant sequence complementarity to Gypsy repeats through sequence based alignment.

Figure 4. Depiction of various noncoding RNAs where distinct mutations resulted in microRNA formation. The distribution of 273 noncoding RNA miR alignments indicating non-transposable element origins identified in our analyses are depicted. In all, 60 corresponded to snoRNAs, 16 to scaRNAs, 41 to rRNAs, 84 to tRNAs, 11 to snRNAs, 38 to various ribozymes and 23 to other forms of noncoding RNAs such as antisense RNAs, long noncoding RNAs, tmRNAs, and sRNAs.
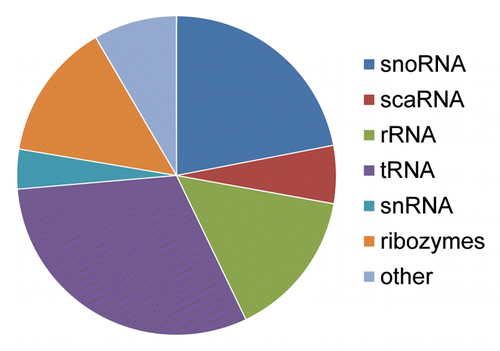
Figure 5. Formation of a miR through tRNA mutation. (A) Structural diagram illustrating the potential effects of a single point mutation to tRNA secondary structure. The most thermodynamically stable conformations of two tRNA sequences are shown. The two sequences resulting in the distinct structural conformations are identical except that the uracil at position 50 in the endogenous tRNA shown on the left has been replaced by a cytosine at position 50 on the right. Secondary structures and thermodynamic stabilities were computed using Mfold.Citation37 (B) Caenorhabditis elegans miR-4937 alignment to the RFAM data set. All repetitive elements and noncoding RNAs (open rectangles) occurring within 500 bp (5′ and 3′) have been included in the scale diagram as depicted in . While we find numerous loci arising by the mechanism depicted in , we find others (like miR-4937) do not. Significantly aligning to a C. elegans tRNA (tRNAArgTCG), we propose an additional mechanism (point mutation(s) resulting in an alteration of normal tRNA secondary structure gave rise to pre-miR-4937.
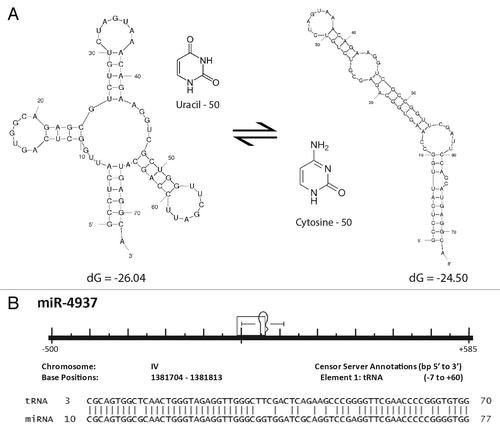
Figure 6. Alignments of miRs with predicted targets. Illustration shows a predicted miR target 3′UTRs on top, a consensus transposable element in the middle, and the corresponding miR sequence on the bottom. Mature miRs are highlighted in gray. Open boxes indicate perfect seed matches. To qualify as a 3′UTR match alignments were required to 1) contain a perfect seed match, 2) match ≥ 50% of the flanking sequence used in the target query, and 3) occur within a 3′UTR sequence aligning to a miR’s progenitor TE sequence. Vertical lines indicate base identity with the TE consensus sequence. Dotted lines indicate purine/pyrimidine conservation. PAH, Gallus gallus phenylalanine-4-hydroxylase ENSGALG00000012754. PTGIR, Sus scrufa prostaglandin I2 (prostacyclin) receptor ENSSSCG00000026602. RC, reverse complemented
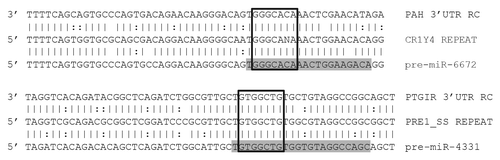
Figure 7. Noncoding RNA miR origins. Alignments showing the high degree of sequence conservation between select miRs (top) and progenitor noncoding RNA (bottom) sequences. miR-1839, cgr-mir-1839 mi0020426; scaRNA, RF00426 SCARNA15 ABDC01642996.1/97-188; miR-6173, hbr-MIR6173 mi0021483; rRNA, RF01959 SSU rRNA AF166114.1/117516-116014; miR-1903, cgr-mir-1903 mi0020435; tRNA, RF00005 tRNA AAHX01000286.1/15293-15364; miR-1291, ggo-mir-1291 mi0020755; snoRNA, RF00410 SNORA2 CEC01039678.1/1523-1387; miR-3473b, mmu-mir-3473b mi0016997; U4, RF00015 U4 AAHX01028334.1/65497-65654.
