Figures & data
Figure 1. (A) Images of the hypothalamic area dissected from wild-type E14.5 mouse brain to generate parental AC1 cells (panels 1–2). The cells were plated in medium supplemented with of bFGF and EGF (schematic in 3). (B–D) Phase-contrast images of AC1 cells taken 5 d after the initial plating in vitro (P0) (B), at passage 1 (P1) (C), and passage 2 (P2) (D). Scale bars: 10 mm (A), 100 μm (B-D).
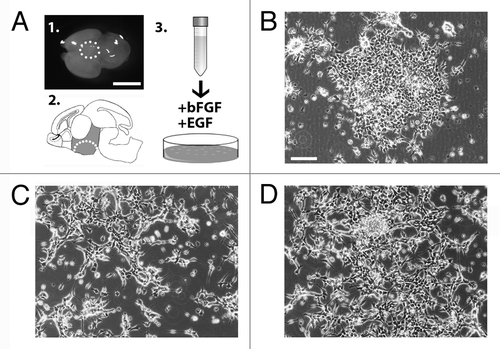
Figure 2. (A–D) Self renewing AC1 cells at (passage 22) immunostained for neural stem cell markers SOX2, nestin, PAX6, vimentin, Olig2, for the radial glia marker RC2 and for markers for neurons (βIII-tubulin) and astrocytes (GFAP). Examples of immunopositive and immunonegative cells are indicated with solid arrowheads and empty arrows, respectively. Nuclei were counterstained with DAPI (A’–D’). (E) RT-PCR analysis on self-renewing AC1 cells revealed the expression of markers for developing hypothalamic progenitors such as Brn2, Arnt2, and Mash1. RNA from E14.5 hypothalamus was chosen as positive control (Ctrl+). The picture is representative of three independent experiments with consistent results. (F) Self-renewing AC1 cells at (passage 22) immunostained for ARNT2 and MASH1. Examples of double immunopositive cells are indicated with a solid arrowhead. Nuclei were counterstained with DAPI (F’). Immunofluorescence images are representative of at least two independent experiments. Scale bar: 50 μm (A–D’, F, and F’).
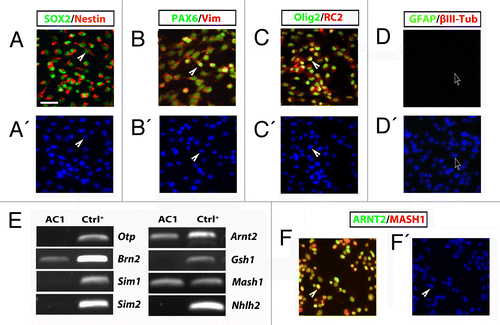
Figure 3. (A–C) Phase-contrast images of stably AC1 cell cultures taken before, in self renewal conditions, (A) and after 3 (B), and 15 (C) days of in vitro differentiation (DIV). Solid arrowheads point at examples of neurites emerging from AC1 cell bodies. (D–F) Immunofluorescence images, representative of at least two independent experiments, of AC1 cells stained for neural precursors (βIII-tubulin, MAP2ab and nestin) and astroglial (GFAP) markers (D–F). Nuclei were counterstained with DAPI. Solid arrowheads point at examples of immunopositive neurites for the indicated markers. (G) RT-PCR on AC1 cells at different time points of differentiation (0, 3, and 15 DIV) revealed changes in the expression of markers for developing hypothalamic neurons with the appearance of transcripts for Otp, Brn2, Sim1, Sim2, and Gsh1. Scale bar: 50 μm (A–F).
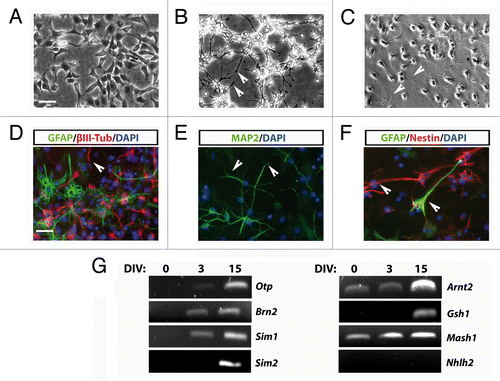
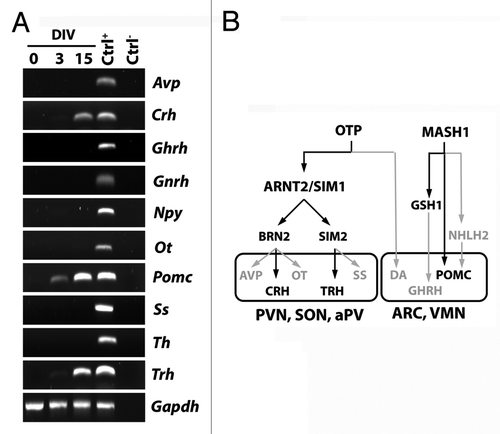
Figure 4. (A) RT-PCR analysis for the expression of hypothalamic peptides and tyrosine hydroxylase (Th), as markers of mature hypothalamic neurons, in AC1 cells at different time points of differentiation (0, 3, and 15 DIV) revealed the presence of Pomc transcript at 3 and 15 DIV and the expression of Crh and Trh at 15 DIV. The picture is representative of three independent experiments with consistent results. Ctrl+, positive control (adult mouse hypothalamus); Ctrl−, PCR negative control (RNA samples not retrotranscribed). Avp, arginine-vasopressin; Npy, neuropeptide Y, Ot, oxytocin; Pomc, proopiomelanocortin; Ss, somatostatin; Th, tyrosine hydroxylase; Gapdh, Glyceraldehyde 3-phosphate dehydrogenase. (B) Schematic representation of the transcription factors suggested to be required for the development of hypothalamic nuclei (hypothalamic transcription factor code) and the differentiation of neuroendocrine neurons in mouse. In gray those not expressed, and as well as the pathways not activated, in AC1 cells under the neuronal differentiation protocol. PVN, paraventricular nucleus; aPV anterior periventricular nucleus,; SON, supraoptic nucleus; ARC, arcuate nucleus; VMN, ventromedial nucleus; DA, dopaminergic neurons of the A11 group.