Figures & data
Figure 1 Expression of lamin A in CRC cells causes increased cell motility. (A) Whole cell extracts of SW480/lamA and SW480/cntl cells were resolved on a 10% SDS-PA GE gel, transferred to nitrocellulose and probed with Jol2 (anti-lamin A/C) antibody. Actin antibody was used as a loading control. (B) Densitometric analysis of endogenous lamin A expression in SW480/lamA cells relative to SW480/cntl cells. Images were taken with Fujifilm Intelligent Dark Box II and relative densities were measured with Image J software. (C) Densitometric analysis of GFP-lamin A plus lamin A expression in SW480/lamA cells relative to lamin A expression in SW480/cntl cells were performed as above. Error bars in (B and C) show the standard error calculated in replicate experiments, *p < 0.05. (D) Representative phase-contrast images of the start and end time points of a cell wounding assay, in which scratch wounds were made in 100% confluent cultures of SW480/lamA or SW480/cntl cells and wound closure was subsequently measured over 24 h using a Zeiss Cell Imager. Scale bars = 20 µm. (E) The mean distance moved by cells over 24 hours in scratch-wounding assays was calculated from phase-contrast images. In each experiment, three wound locations were chosen for each of three biological replicates and images were taken every 15 minutes for 24 hours. Error bars represent the standard error calculated from the biological replicates. SW480/lamA cells moved 20% further than SW480/cntl cells in 24 hours (p < 0.05). (F) Immunoblot of whole cell extracts from SW480/lamA cells transfected with si-laminA (si-l) or scrambled negative control siRNA (si-c), were resolved on a 10% SDS-PA GE gel and probed with Jol2 (anti-lamin A/C) antibody. Anti-actin antibody was used as a loading control. Cell pellets were harvested at 24, 48, 72, 96 and 120 hours post transfection with siRNA. (G) Representative phase-contrast images of the start and end time points of a cell wounding assay, in which SW480/lamA cells were grown to 100% confluency and wounded at 96 hours post-transfection with si-lamin A or si-cntl. Wound closure was measured over 24 h using a Zeiss Cell Imager. Scale bars = 20 µm. (H) The mean distance moved by cells in 24 hours was calculated from the phase-contrast images. In each experiment, four wound locations were chosen for each of three biological replicates and images were taken every 15 minutes for 24 hour. Error bars represent the standard error calculated from the biological replicates performed for each siRNA type. Si-cntl cells moved 40% further than si-laminA cells over 24 h (p < 0.0005).
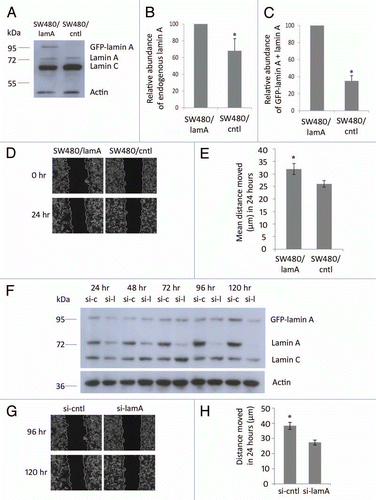
Figure 2 The membrane-bound protein vinculin is lost from detergent/high salt resistant N/CSK during biochemical fractionation of SW480 cells, whereas cytoskeletal proteins remain mostly insoluble. Pellets and supernatants were prepared from SW480/lamA and SW480/cntl cells at 70% confluency as described in materials and methods. Each fraction was resolved on 12% SDS-PAGE gels and transferred to nitrocellulose. Blots were probed with anti-actin (A and B), anti-α-tubulin (C and D), anti-keratin 18 (E and F) or anti-vinculin (G and H) antibodies.
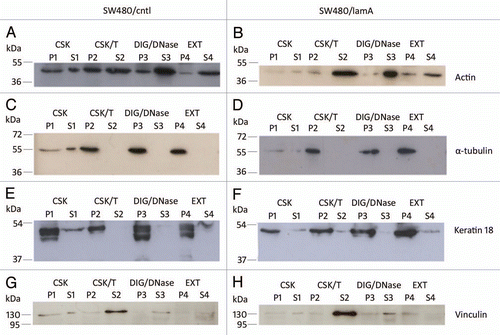
Figure 3 2D mini-format gels confirm reproducibility of the final cytoskeleton fraction prepared from SW480/lamA and SW480/ctl cells. Detergent/high salt resistant N/CSKs were isolated from SW480/lamA and SW480/cntl cells in six independent experiments. Samples were acetone precipitated and resolubilised in lysis buffer. 100 µg of protein was loaded using in-gel rehydration into 7 cm pH 4–7 IPG strips. Proteins were resolved in the first dimension using isoelectric focusing and in the second dimension using 10% mini-format SDS-PA GE gels. The resolved proteins were visualised using Coomassie blue staining. Images show mini-format gels run with replicate samples from SW480/cntl cells (A, C, E, G, I and K) and from SW480/lamA cells (B, D, F, H, J and L).
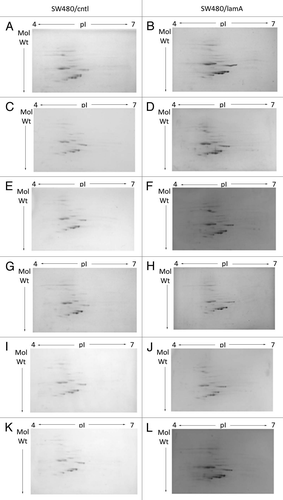
Figure 4 Differences in protein abundances in detergent/high salt resistant N/CSK isolated from SW480/lamA and SW480/cntl cells (A) 2-D DIGE gel comparing detergent/high salt resistant N/CSK from SW480/lamA and SW480/cntl cells. Arrows represent protein spots selected for analysis by MALDI-ToF-ToF. Differentially expressed spots were excised from a high protein load pick gel. Spots are annotated with their spot number for cross-referencing with Tables 1 and 2. Spots circled in red represent proteins over-represented in the fraction from SW480/lamA cells. Spots circled in green represent proteins under-represented in the fraction from SW480/lamA cells. (B) Cropped 2-D DIGE gel as above, annotated to show the identities of the proteins determined by MALDI-ToF-ToF.
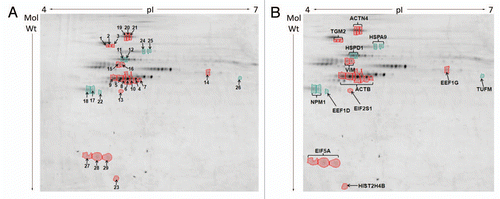
Table 1A Proteins over- and under-represented in detergent/ high salt resistant N/CSKs of SW480/lamA cells; (A) Proteins over-represented in the cytoskeleton preparations of SW480/lamA cells
Table 1B Proteins over- and under-represented in detergent/ high salt resistant N/CSKs of SW480/lamA cells; (B) Proteins under-represented in the cytoskeleton preparations of SW480/lamA cells