Figures & data
Figure 1 Geminin Protein Accumulates After Entry Into S Phase. CHO cells were synchronized in mitosis by shake-off and released into the cell cycle for the indicated times. Either whole cell extracts (WCE ) or a chromatin-containing cell fraction (chromatin) was subjected to western blotting with the indicated antibodies. Cell cycle hallmarks discussed in the text are indicated above the figure.
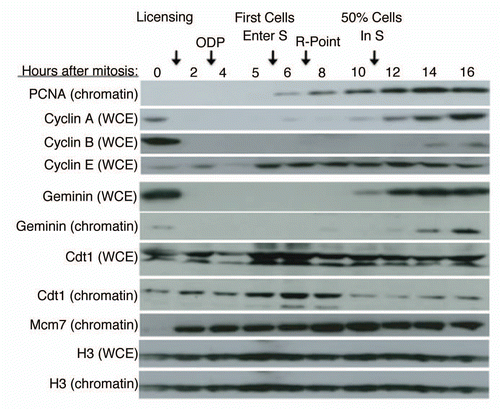
Figure 2 A heterologous system for the identification of licensing activities. (A) Description of licensing assay protocol (see text and methods for details). (B) Various concentrations of sperm chromatin were added to soluble high-speed Xenopus extract in Step 1, followed by the addition of sufficient geminin-supplemented complete (low-speed) extract to maintain a constant sperm template concentration in Step 2 of 13.3 ng/µl. The relative fraction of input DNA synthesized was plotted as a function of original template concentration in Step 1. This experiment identifies the Step 1 template concentration at which licensing components (e.g., Mcm2–7) become limiting for DNA synthesis. 40 ng/µl was used in all subsequent experiments. (C) Immediately following Step 1 from (B), sperm chromatin was isolated from aliquots of the reaction by centrifugation through a sucrose cushion. Chromatin bound proteins from equivalent amounts of sperm DNA from each reaction were subjected to immuno-blotting to detect the amount of chromatin-loaded Mcm proteins. (D) In Step 1, soluble high-speed Xenopus egg extract was replaced with either Buffer (Buf) or soluble extracts from CHO cells at different times during the cell cycle (M = mitosis or cells at shake-off; Early G1 = 2 hours after mitosis; Late G1 = 7 hours after mitosis; S = S phase; cells released from mitosis into aphidicolin for 16 hours and released from the aphidicolin block for one hour). CHO extracts cannot license Xenopus sperm chromatin on their own, regardless of the time during the cell cycle at which they are prepared. (E) Soluble high-speed Xenopus egg extracts were depleted of ORC1, Cdc6, Mcm3 or Cdt1 and Step 1 of the licensing reaction was performed with mixtures of depleted extract and either Buffer (Buf) or CHO cell extracts (Xenopus: Mammalian extract ratio 1:3) prepared at the indicated times during the cell cycle as in (D). As positive control reactions, depleted Xenopus extracts were supplemented with partially purified fractions of Xenopus egg extract (Crude Xen.: PEG-M for Mcm3 depleted extracts and PEG-B for all others; reference Citation18) and all reactions were quantified relative to this positive control. (F) Soluble hamster extracts used in (E) were subjected to immuno-blotting to examine the level of hamster Cdt1 and Mcm proteins.
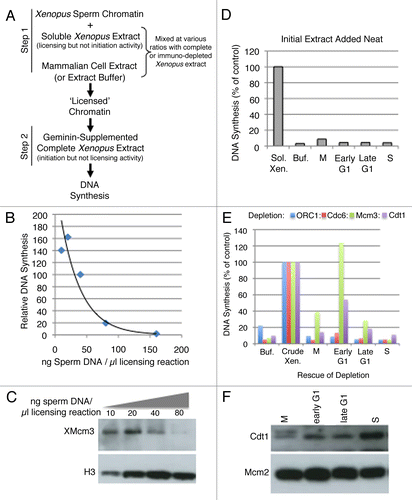
Figure 3 Inhibition of licensing by hamster extracts. (A) Various ratios of high-speed Xenopus extract: mammalian extract or buffer (Buf.) were mixed with 40 ng/µl sperm chromatin during Step 1 of the licensing reaction, followed by the addition of sufficient geminin-supplemented complete (low-speed) extract to maintain a constant sperm template concentration in Step 2 of 13.3. ng/µl, as in . Licensing inhibition is shown as a percentage of the DNA synthesis seen relative to the maximum value when the assay was performed with high-speed Xenopus extract:buffer = 3:1. (B) The experiment shown in (A) was repeated with 3 independent batches of Xenopus and CHO extracts. For M and S phase reactions, the “Optimal Ratio” of Xenopus:Mammalian extract was determined as the minimum amount of hamster extract to give maximal inhibition, which was 1:1 for M phase and 3:1 for S phase. For G1 phase inhibitory activity, the “Optimal Ratio” was the ratio to detect the maximum licensing inhibition for late G1 relative to early G1 extracts, which was 1:3.
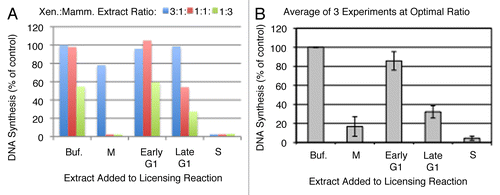
Figure 4 Nature of licensing inhibitor in M phase and S phase hamster extracts. (A and C) Step 1 of the licensing assay was performed with highspeed Xenopus extract mixed with buffer alone (grey) or with M phase (A; ratio of 1:1) or S phase (C; ratio of 3:1) CHO extract (white). Prior to mixture, buffer or CHO extracts were supplemented with the Cdk inhibitor 6-DMAP (3 mM), a geminin-neutralizing fragment of Cdt1 (amino acids 193–447; Con7, 5 ng/µl), or both inhibitors simultaneously. DNA synthesis in Step 2 is expressed as a percentage of that obtained with buffer alone. Shown are the means of three experiments with independent extract batches and error bars show standard deviation. (B and D) Xenopus sperm chromatin was incubated with high-speed Xenopus extract mixed with buffer (+Buffer) or hamster M-phase (B) or S-phase (D) extract supplemented with inhibitors as in (A and C) except that Roscovitine (rosco, 40 µM) was substituted for 6-DMAP as a more potent and specific inhibitor of Cdk activity. Following the 25 min Step 1 incubation period, sperm chromatin was isolated and subjected to immuno-blotting to evaluate Mcm protein loading.
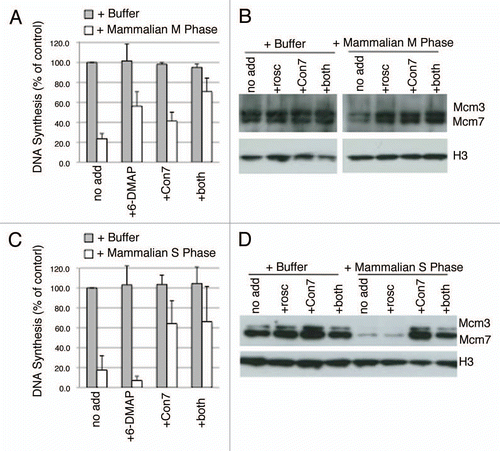
Figure 5 Nature of licensing inhibitor in late G1 hamster extracts. (A) Similar to and C, Step 1 of the licensing assay was performed with high-speed Xenopus extract mixed with buffer alone (Buf) or with extracts from CHO cells synchronized at the indicated times after mitosis at a 1:3 ratio. Prior to mixture, buffer or CHO extracts were supplemented with 6-DMAP (red), or Cdt1193–447 (green). DNA synthesis in Step 2 is expressed as a percentage of that obtained with buffer alone. Shown are the results of two independent assays and the standard deviation. Below the graph is an immuno-blot showing the Mcm2 and geminin levels in the soluble hamster extracts from each time point, as well as M and S phase from the same synchronized cells. (B) Xenopus sperm chromatin was incubated with high-speed Xenopus extract mixed with buffer (+Buffer) or extracts from CHO cells synchronized at the indicated times after mitosis. Following the 25 min Step 1 incubation period, sperm chromatin was isolated and subjected to immuno-blotting to evaluate the relative amounts of Mcm loading after incubation with extracts from each G1 phase time point (A). In this experiment, histones were detected by coomassie staining. (C) Prior to collection, aliquots of each of the cell preparations from (A) were pulse labeled with BrdU and the percentage in S phase cells was evaluated. (D) Step 1 of the licensing reaction was performed with high-speed Xenopus extract mixed at a 1:1 ratio with buffer (+Buffer) or extracts from CHO cells synchronized either 2 hours after mitosis (Early G1 Phase) or after release from aphidicolin as in – (S Phase), as well as combinations of these two CHO cell extracts mixed at the indicated ratios. This demonstrates that 10–33% contamination of S-phase cells would be needed to detect licensing inhibition. (E) Similar to and C, Step 1 of the licensing assay was performed with high-speed Xenopus extract mixed at a ratio of 1:3 with buffer alone (grey) or with extracts from CHO cells synchronized 7 hours after mitosis (Late G1 Phase; white). Prior to mixture, buffer or CHO extracts were supplemented with the Cdk inhibitor 6-DMAP , Cdt1193–447 (Con7), or both inhibitors simultaneously. DNA synthesis in Step 2 is expressed as a percentage of that obtained with buffer alone. Shown are the means of three experiments with independent extract batches and error bars show standard deviation. (F) Similar to and D, Xenopus sperm chromatin was incubated with high-speed Xenopus extract mixed at a ratio of 1:3 with buffer alone (+Buffer) or extracts from CHO cells synchronized 7 hours after mitosis (+Mammalian G1 Phase) supplemented with inhibitors as in (E) except that Roscovitine (rosco) was substituted for 6-DMAP as a more potent and specific inhibitor of Cdk activity, as in and D. Following the 25 min Step 1 incubation period, sperm chromatin was isolated and subjected to western blotting to evaluate Mcm protein loading. Neither Roscovitine nor Cdt1193–447 (Con7) could recover Mcm loading to levels seen with Buffer alone.
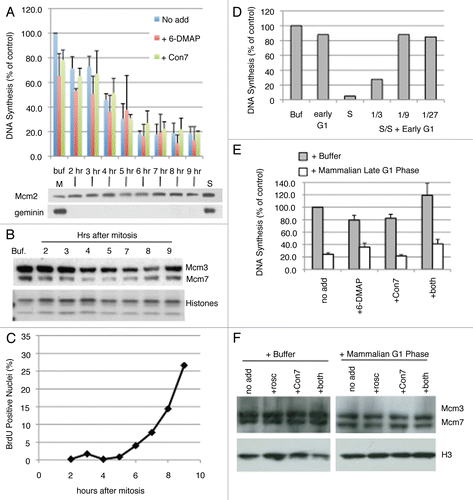
Figure 6 Drug effect on late G1 licensing inhibition. (A) Step 1 of the licensing assay was carried out mixing high-speed Xenopus extract at a 1:3 ratio with either Buffer (Buf) or soluble extracts from CHO cells synchronized at the indicated times after mitosis. Cells were treated with the indicated inhibitors (cycloheximide, CHX 50 µg/ml; MG132, 10 µM; roscovitine, 40 µM) starting from 2 hours after mitosis. Shown are the average of two independent assays and the standard deviation. (B) Progression into S phase for each of the CHO cell cultures used to prepare extracts in (A) was monitored by BrdU pulse labeling as in .
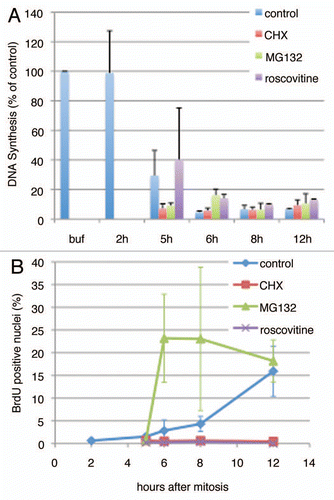
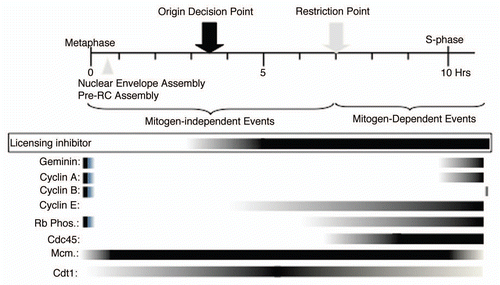
Figure 7 Summary. The timing of appearance of the licensing inhibitory activity identified in this report is placed into the context of the well-studied G1 phase hallmarks.