Figures & data
Figure 1. The evolution of aging and dietary effects on lifespan. (A) Positive and antagonistic pleiotropy theories suggest that alterations conferring advantages in early life respectively trigger beneficial and deleterious effects at the post-reproductive age. (B) Generalized relationships between diets and lifespan.
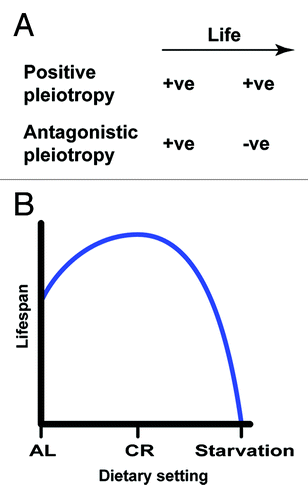
Table 1. The effect of CR on various species
Figure 2. Calorie restriction (CR) increases lifespan by increasing genomic stability. Stability of DNA is maintained by increasing DNA repair pathways and controlling repetitive DNA loci. DNA repair pathways controlled by CR include base excision repair (BER), nucleotide excision repair (NER) and non-homologous end-joining (NHEJ). At the repetitive DNA loci, CR prevents deleterious recombination at the rDNA repeats, increases telomeric silencing and maintains telomere length to increase lifespan. CR can also increase genomic stability by lowering the production of reactive oxygen species (ROS) as well as promoting the function of antioxidants (e.g., superoxide dismutase enzymes). CR may decrease ROS production by increasing mitochondrial efficiency or by decreasing mitochondrial membrane potential.
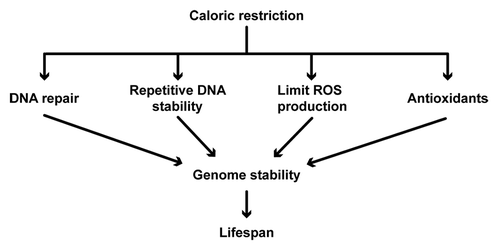
Figure 3. Calorie restriction (CR) influences reactive oxygen species (ROS) production through a delicate balance. When CR decreases mitochondrial membrane potential and increases antioxidant expression, ROS production is reduced relative to ad libitum and lifespan may be increased (top scale). If either of these factors is absent, ROS production will be increased relative to ad libitum and lifespan will be decreased (bottom scales).
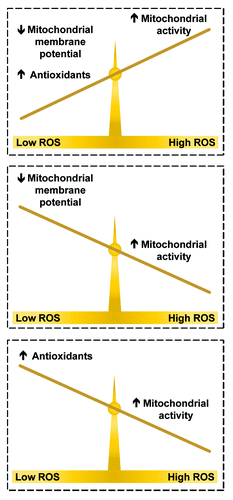
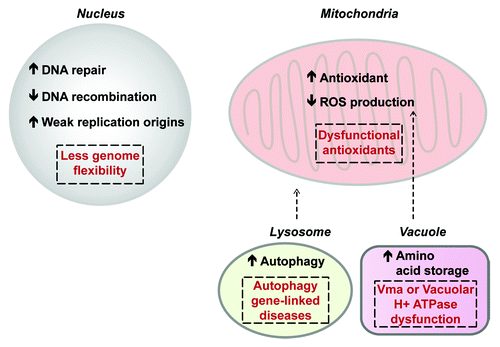
Figure 4. Summary of the effects of calorie restriction (CR) on various cellular components. CR increases DNA repair, promotes telomere length, decreases recombination particularly within repetitive DNA loci, and may also ensure the function of weak DNA replication origins. Taken together, the net effect of these changes is a decreased genomic flexibility and this may in turn prevent cells from efficiently adapting to various stress conditions (nuclear dashed box). CR-dependent hyperactivation of DNA repair processes early in life may disrupt the balance between DNA repair and telomere maintenance. With regard to mitochondrial processes, CR increases antioxidant function and lowers membrane potential to lower ROS production even in the presence of CR-dependent mitochondrial hyperactivity. However, in settings where CR-dependent increases in mitochondrial activity are not mitigated by other processes such as in superoxide dismutase mutant, CR increases ROS-dependent damage (mitochondrial dashed box). In the vacuole, CR lowers pH, which promotes amino acid storage and may help lower mitochondrial membrane potential and overall ROS levels. Vacuolar defects can cause CR to have a net negative effect on lifespan (dashed box inside vacuole). In the lysosome, CR promotes an increase in autophagy, which can help eliminate old organelles, including dysfunctional mitochondria. This helps mobilize cellular energy stores and limit toxicity caused by defective organelles. Mutations within autophagy genes are linked to a large number of clinical settings and this can in turn cause partial CR-dependent activation of autophagic processes triggering toxicity and lowering lifespan (dashed box within lysosome).