Figures & data
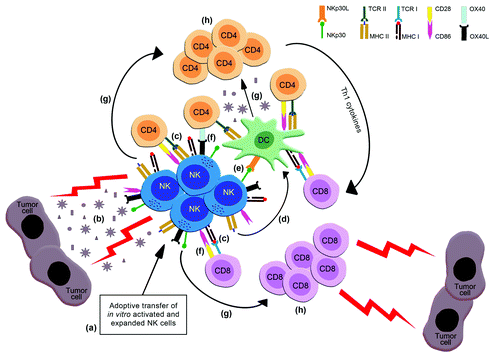
Figure 1. Adoptive transfer of ex vivo activated and expanded NK cells can act as tumor-specific active immunotherapy, inducing long lasting anti-tumor T cell responses. NK cells can be effectively activated and expanded in vitro by cytokines in combination with GCs and/or other “stress” signals (a). Adoptively transferred activated NK cells can lyse tumor cells thus leading to the release/ production of apoptotic bodies/TAAs (b). Such tumor-derived material will be processed and presented to CD4 and CD8 T cells (i) directly, by the activated NK cells which express MHC class I and class II molecules (c) or (ii) indirectly, through NK cell-mediated activation and polarization of DCs. This can be achieved by activated NK cell-produced cytokines (IFNγ, TNFα, GM-CSF) (d) or by direct cell-to-cell contact (i.e., NKp30-NKp30L) (e). Furthermore, activated NK cells expressing costimulatory molecules (e.g., CD86, OX40L, etc) can provide efficient costimulation to T cells (through CD28, OX40, etc) (f). The concerted action of cytokines produced by the activated NKs and DCs (IFNγ, IL-12) (g) will support Th1 responses. The effective priming and restimulation of T cells by NK cells and/or DCs will induce the proliferation and differentiation of CD4+ and CD8+ into effector/memory and central memory cells that can confer long lasting antitumor immunity (h). Improvement of these responses can be achieved through inhibition of immunomodulatory mechanisms (e.g., Treg cell and MDSC elimination/ inhibition, anti-CTLA4 or anti-PD1 targeting etc) as applied in other vaccination strategies (e.g., peptide vaccines, whole cell vaccines, DC-based vaccines etc.).