Figures & data
Figure 1. Clinical efficacy and overall survival of melanoma patients treated with temolozomide. (A) Magnetic resonance imaging (MRI) scans from a patient undergoing a partial response and demonstrating an almost complete response in a brain lesion after 6 mo of temolozomide (TMZ) treatment. (B) Kaplan-Meier plot of progression-free survival (PFS) for all patients included in this study (n = 40). Median PFS was 260 d. PFS was defined as the time intervening between the first dose of TMZ until disease progression or death from any cause. When no progression or death occurred, data were censored at the date of last adequate disease assessment. (C) Duration of TMZ treatment for all enrolled patients. The duration of treatment was defined as time intervening between the first and the last dose of TMZ. The mean treatment duration was 173 d (95% CI = 135.4–210.3), corresponding to ~5 cycles of TMZ treatment.
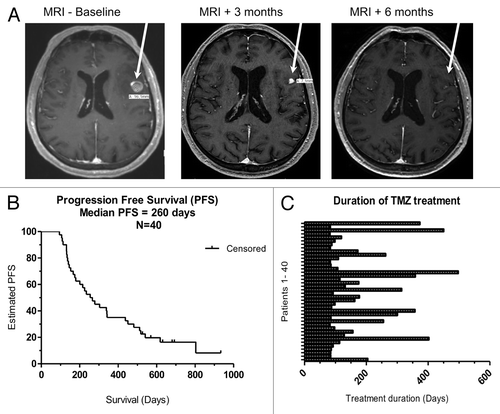
Figure 2. Lymphopenia induced by temolozomide in melanoma patients. Lymphocyte counts on freshly drawn blood samples (as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first, second and 3rd cycles of chemotherapy. **p < 0.01; ***p < 0.001 (paired Student's t-test).
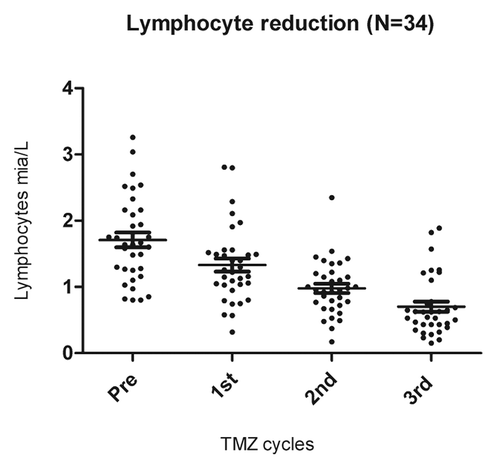
Figure 3. Alterations of the frequency T-cell subsets as induced by temolozomide in melanoma patients. (A–D) Frequency of T cell subpopulations on frozen/thawed peripheral blood mononuclear cells (PBMCs, as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first and second cycles of chemotherapy: *p < 0.05; NS = non-significant (paired Student's t-test).
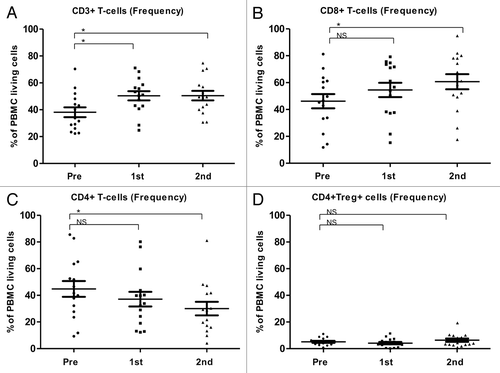
Figure 4. Alterations of the absolute abundance of T-cell subsets as induced by temolozomide in melanoma patients. (A–D) Absolute T-cell counts on frozen/thawed peripheral blood mononuclear cells (PBMCs, as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first and second cycles of chemotherapy. *p < 0.05; NS = non-significant (paired Student's t-test).
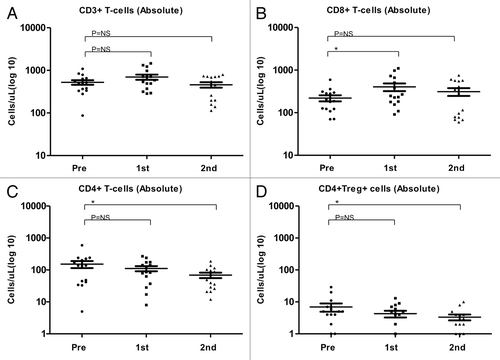
Figure 5. Correlation between temolozomide-induced lymphopenia and clinical parameters. (A) Correlation between the grade of lymphopenia (as determined on freshly drawned blood samples) and the response of n = 34 melanoma patients to 3 cycles of temolozomide (TMZ). p = 0.01 (Fischer’s exact test). Lymphocyte counts were done directly on. (B) Progression-free survival (PFS) of lymphocyte responders (defined as individuals with lymphocyte counts ≤ 0.7 × 109 cells/L, n = 23) and lymphocyte non-responders (defined as individuals with lymphocyte counts > 0.7 × 109 cells/L, n = 11). The Kaplan-Meier estimate demonstrates a non-significantly (p = 0.2, Log-Rank test) prolongation in median PFS in lymphocyte responders (303 d) vs. lymphocyte non-responders (200 d).
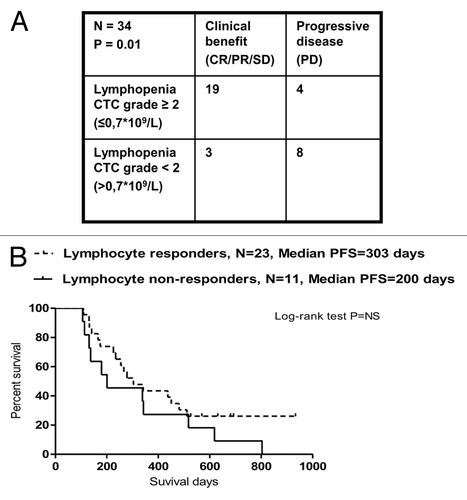
Figure 6. CD8+ T-cell subsets in melanoma patients responding or not to temolozomide. (A) CD8+/CD4+ T-cell ratio (as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first and second cycles of chemotherapy to clinical responders, exhibiting either complete response (CR), either partial response (PR) or stable disease (SD), and clinical non-responders, exhibiting progressive disease (PD). (B) Frequency of naïve CD8+ T cells (as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first and second cycles of chemotherapy. *p < 0.05; NS = non-significant (paired Student's t-test). (C) Frequency of differentiated CD8+ T cell (Tcm plus Tem plus Terma) (as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first and second cycles of chemotherapy. *p = 0.02; NS = non-significant (paired Student's t-test).
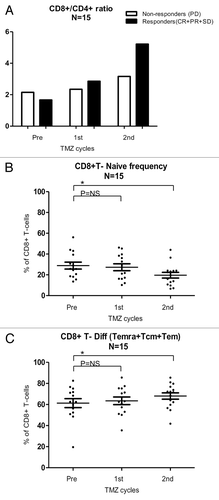
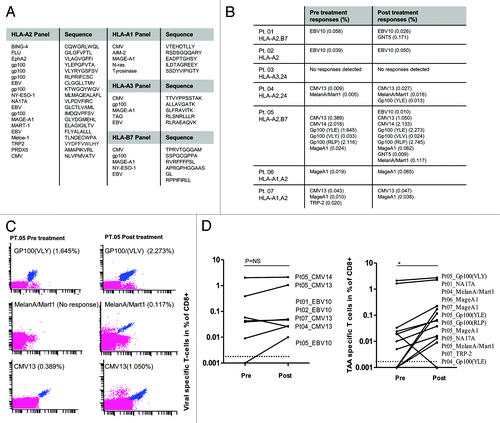
Figure 7. Tumor-associated antigen- and viral antigen-specific T-cell responses in melanoma patients receiving temolozomide. (A) Peptide panel used for screening T-cell responses in patients with tissue types HLA-A1, A2, A3 and B7. (B) Responses to viral and tumor-associated antigens evaluated before and after temolozomide (TMD) chemotherapy in n = 7 melanoma patients. (C) Examples of pre and post-chemotherapy T-cell responses in-patient 05, as detected by flow cytometry with MHC multimers. CD8+ T-cell responses were gated on living single CD8+ T cells (mean number of CD8+ cells for all experiments = 73,655; range = 8056–238,576). MHC multimer positive populations are depicted in blue, from the top recognizing (1) Gp100(VLY), labeled by PE and Q655, (2) MelanA/Mart1, labeled by Q585 and Q605 and (3) CMV13, labeled by Q605 and Pe-Cy7. (D) Viral antigen- and tumor antigen-specific responses pre- and post-TMZ chemotherapy. *p = 0.04; NS = non-significant (paired Student's t-test). The dotted line represents the detection limit = 0.002% CD8+ T cells.