Figures & data
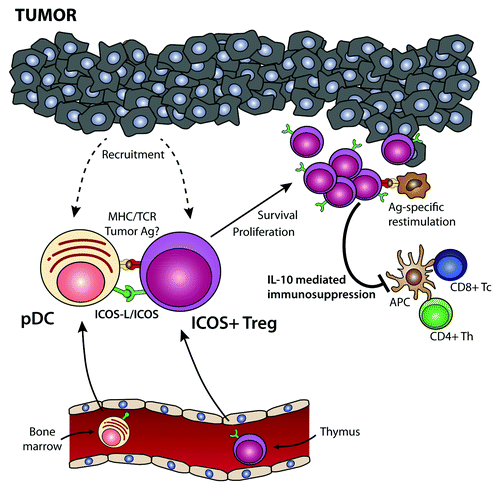
Figure 1. Role of plasmacytoid dendritic cells and ICOS+ regulatory T cells in tumor immunosuppression. Bone marrow-derived plasmacytoid dendritic cells (pDCs) and thymic-derived ICOS+FOXP3+ regulatory T cells are recruited from the circulation into the tumor microenvironment. Tumor-infiltrating pDCs express high levels of the ICOS ligand (ICOSL), which co-stimulates ICOS+FOXP3+ regulatory T cells in the context of tumor-associated antigen presentation by pDCs or bystander antigen-presenting cells (APCs). This drives the activation and proliferation of ICOS+FOXP3+ regulatory T cells, leading to a preferential accumulation of this regulatory T-cell subset within the tumor microenvironment. Upon re-encounter with the tumor-associated antigen, ICOS+FOXP3+ regulatory T cells secrete interleukin (IL)-10, hence suppressing the effector functions of tumor-specific CD4+ and CD8+ T cells. Thus, the infiltration of neoplastic lesions by pDCs favors the establishment of an immunosuppressive microenvironment via the activation and expansion of ICOS+FOXP3+ regulatory T cells, de facto favoring disease progression.