Figures & data
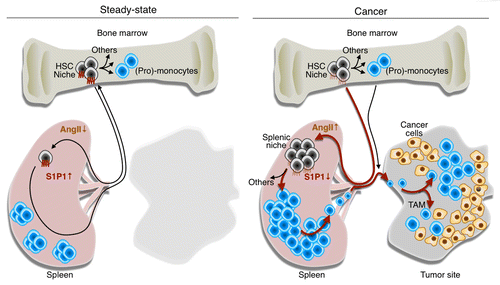
Figure 1. Tumor remote control of hematopoietic stem cells and macrophagic progenitors through angiotensin II. In steady-state conditions, hematopoietic stem cells (HSCs) and macrophagic progenitors typically reside in bone marrow niches and generate (pro-)monocytes. A small number of HSCs constantly enter the circulation, extravasate at distant sites and migrate through peripheral tissues, including the spleen. Under normal conditions, these cells can re-enter the blood and ultimately, return to their niches in the bone marrow. The process of cell recirculation is controlled at least in part by a chemotactic gradient of sphingosine-1-phosphate (S1P) that is established between peripheral tissues, lymphatics and the blood, where S1P concentrations is the highest. Thus, in steady-state conditions, only a few HSCs can be found in the spleen. Multiple types of cancer including lung adenocarcinomas produce angiotensin II (AngII), which directly signals through the AGTR1A receptor expressed on HSCs. This induces the downregulation of the S1P receptor 1 (S1P1), hence reducing the ability of HSCs to sense the S1P gradient. In these conditions, HSCs accumulate in the spleen where they give rise to monocytes. By continuously producing monocytes, the spleen can contribute TAMs throughout tumor progression. Thus, tumors can directly exploit the endocrine system to promote tumor progression. Drugs that interfere with the AngII pathway (or perhaps with S1P1 signaling) may hence become therapeutic options for treating lung cancer patients in whom this pathway is elevated.