Figures & data
Figure 1. OCT4-specific IP-10 production in peripheral blood mononuclear cells from healthy subjects. (A) Freshly isolated peripheral blood mononuclear cells (PBMCs) were labeled for intracellular interferon γ (IFNγ)-inducible protein 10 (IP-10). (B and C) Alternatively, PBMCs were stimulated with an immunoreactive OCT4-derived peptide for 48 h, followed by the assessment of intracellular IP-10 by flow cytometry (B) or IP-10 secretion by ELISA (C). Non-stimulated PBMCs were included as a negative control. No brefeldin A was added to the culture, and a minimum of 1×105 cells was analyzed.
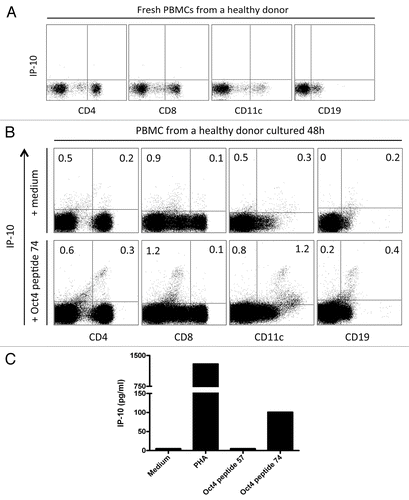
Figure 2. IP-10 production by OCT4-specific T cells. (A and B) MACS-sorted CD4+ and CD8+ cells were stimulated with autologous dendritic cells (DCs) loaded as indicated, and after 2 d of co-culture interferon γ (IFNγ)-inducible protein 10 (IP-10) secretion was assessed by ELISA. (A) IP-10 production by T cells exposed to autologous DCs loaded with an immunoreactive OCT4-derived peptide (representative data from one donor). (B) IP-10 production by T cells exposed to autologous DCs loaded with the OCT4 mRNA (representative data from the same donor).
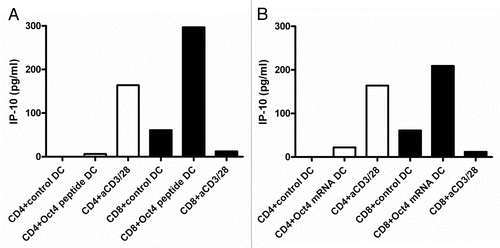
Figure 3. Detection of OCT4-reactive CD4+ T cells. MACS-sorted CD4+ cells were stimulated with autologous dendritic cells (DCs) loaded with an immunoreactive OCT4-derived peptide. CD4+ cells stimulated with anti-CD3/anti-CD28 beads and CD4+ cells exposed to unloaded DCs served as a positive and negative control condition, respectively. Results from 2 representative donors showing interferon γ (IFNγ) production (on day 3) and antigen-dependent CD4+ T-cell proliferation (on day 6) are reported. CD4+ cells from both donors did not exhibit high IFNγ production by in response to OCT4-derived peptide-loaded DCs (p > 0.05), while cells from one donor underwent a significant proliferative response (p < 0.05).
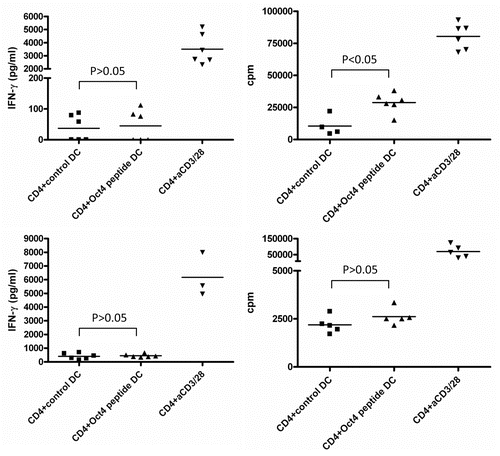
Figure 4. Detection of OCT4-reactive CD8+ T cells. (A–C) MACS-sorted CD4+ cells were stimulated with autologous dendritic cells (DCs) loaded with an immunoreactive OCT4-derived peptide. CD4+ cells stimulated with anti-CD3/anti-CD28 beads and CD4+ cells exposed to unloaded DCs served as a positive and negative control condition, respectively. (A) Results from one representative donor showing no significant interferon γ (IFNγ) production (on day 3) and no antigen-dependent CD8+ T-cell proliferation (on day 6) (p > 0.05) are reported. (B) After 12 d of co-culture, a second stimulation was performed with -derived peptide-loaded DCs. Thereafter, peptide-specific CD8+ T cells were analyzed for their cytotoxic functions by intracellular cytokine staining and flow cytometry. The expression of activation markers by cells from one representative healthy donor is shown, together with the gating strategy. (C) Results from one representative donor showing the mobilization of CD107a and the expression of IFNγ and tumor necrosis factor α (TNFα) by the same cells [gating strategy as in (B)] are shown.
![Figure 4. Detection of OCT4-reactive CD8+ T cells. (A–C) MACS-sorted CD4+ cells were stimulated with autologous dendritic cells (DCs) loaded with an immunoreactive OCT4-derived peptide. CD4+ cells stimulated with anti-CD3/anti-CD28 beads and CD4+ cells exposed to unloaded DCs served as a positive and negative control condition, respectively. (A) Results from one representative donor showing no significant interferon γ (IFNγ) production (on day 3) and no antigen-dependent CD8+ T-cell proliferation (on day 6) (p > 0.05) are reported. (B) After 12 d of co-culture, a second stimulation was performed with -derived peptide-loaded DCs. Thereafter, peptide-specific CD8+ T cells were analyzed for their cytotoxic functions by intracellular cytokine staining and flow cytometry. The expression of activation markers by cells from one representative healthy donor is shown, together with the gating strategy. (C) Results from one representative donor showing the mobilization of CD107a and the expression of IFNγ and tumor necrosis factor α (TNFα) by the same cells [gating strategy as in (B)] are shown.](/cms/asset/1c1711b2-446f-457c-b04a-37f2dd3cb668/koni_a_10924271_f0004.gif)
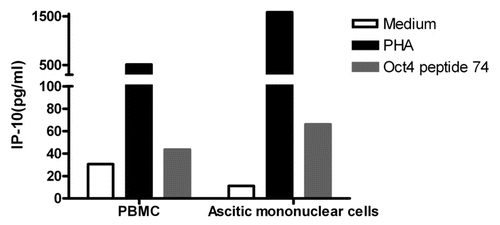
Figure 5. Immunity to OCT4 in ovarian cancer patients. Peripheral blood mononuclear cells (PBMCs) and ascitic fluid-derived mononuclear cells from the same patient were cultured in the presence or in the absence of an immunoreactive OCT4-derived peptide for 48 h, followed by the quantification of interferon γ (IFNγ)-inducible protein 10 (IP-10) secretion in co-culture supernatants. Phytohemagglutinin PHA-stimulated cells were used as a positive control. Results from one representative patients are shown.