Figures & data
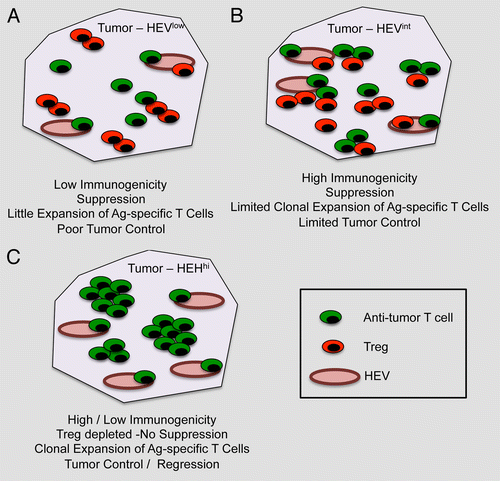
Figure 1. Impact of high endothelial venules on tumor progression. (A) In the case of poorly immunogenic tumors, high endothelial venules (HEVs) are not abundant (HEVlow) and the neoplastic lesion is infiltrated by both conventional T cells and Foxp3+ regulatory T cells (Tregs). In this setting, antitumor T cells have limited chances to expand and tumor growth remains virtually unaffected.(B) When tumor are highly immunogenic, the number of intratumoral HEVs is slightly increased (HEVint), resulting in a relatively more intense recruitment of conventional T cells and Tregs. In this case, tumor-specific T cells may undergo some expansion, yet the presence of Tregs limits their antineoplastic effects. (C) The depletion of Tregs not only limits immunosuppression but also greatly increase the number of intratumoral HEVs (HEVhi). This results in the elicitation of robust antitumor immune responses that can control disease progression (at least theoretically) irrespective of the immunogenicity of malignant cells.