Figures & data
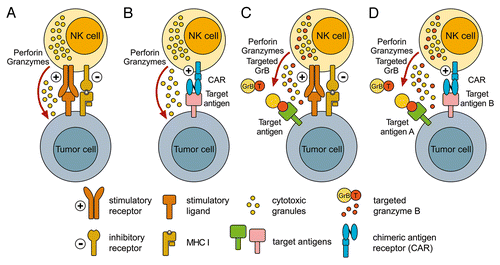
Figure 1. Enhancement of NK-cell antitumor activity by the expression of tumor-specific receptors and cytotoxic fusion proteins. (A) The natural cytotoxicity of natural killer (NK) cells against tumor cells is controlled by signals from stimulatory receptors, such as natural cytotoxicity receptors (NCRs) and NKG2D, and inhibitory receptors, such as inhibitory killer-cell immunoglobulin-like receptors (KIRs). (B) The expression of a chimeric antigen receptor (CAR) specific for tumor-associated cell surface antigens efficiently redirects NK cells to malignant cells, and facilitates their cytolytic activity independently from the activation of endogenous stimulatory receptors. (C) Alternatively, NK cells can be modified to express targeted cytotoxic chimeras such as the pro-apoptotic serine protease granzyme B (GrB) fused to a tumor cell-specific ligand or antibody fragment. Such molecules are stored together with endogenous granzymes and perforin in cytotoxic granules. Upon the activation of endogenous NK-cell receptors, tumor-targeted GrB is released together with perforin and hence can cooperate with natural cytotoxicity mechanisms in the killing of target cells. Nonetheless, insufficient signals via endogenous NK-cell stimulatory receptors can prevent the release of retargeted GrB variants. (D) This issue may be overcome by the co-expression of tumor-targeted GrB derivatives together with a CAR that ensures NK-cell activation even by otherwise resistant cancer cells. In this scenario, CARs and targeted GrB may be directed to the same or distinct tumor-associated cell surface antigens.