Figures & data
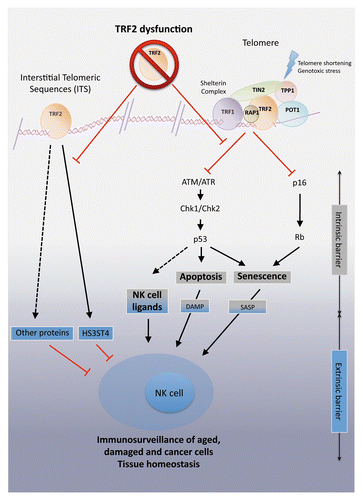
Figure 1. TERF2 controls innate immunosurveillance through cell-intrinsic and cell-extrinsic pathways. Two pathways link telomeres to the activation of natural killer (NK) cells. On the one hand, telomere dysfunction triggers the DNA damage response (DDR), which promotes apoptosis or senescence, constituting a cell-intrinsic barrier against oncogenesis. This can lead to the recruitment of NK cells through a p53-dependent signaling pathway, the release of damaged-associated molecular patterns (DAMPs) or the activation of the senescence-associated secretory program (SASP). On the other hand, the binding of telomeric repeat-binding factor 2 (TERF2) to DNA regions other than telomeres results in the transactivation of heparan sulfate (glucosamine) 3-O-sulfotransferase 4 (HS3ST4). HS3ST4 actually inhibits the recruitment of NK cells. The dysfunction of TERF2, compromising both cell-intrinsic and -extrinsic barriers to carcinogenesis, exerts a positive effect on (NK cell-dependent) cancer immunosurveillance.