Figures & data
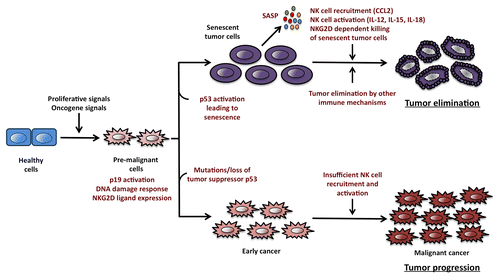
Figure 1. Oncogene-induced senescence promotes immunosurveillance by natural killer cells. The activation of oncogenes and the consequent delivery of proliferative signals to healthy cells generate a pool of pre-malignant cells expressing ligands for the NKG2D receptor (also called killer cell lectin-like receptor subfamily K, member 1, KLRK1, in humans). In this setting, cyclin-dependent kinase inhibitor 2A, isoform 4 (also called p19ARF in mice or p16ARF in humans) and the DNA damage response (DDR) can activate p53 and other oncosuppressive factors that promote cellular senescence. Senescent cells stop proliferating and mobilize an oncosuppressive mechanism mediated by immune cells. The so-called “senescence-associated secretory phenotype” (SASP) is associated with the release of chemokines such as chemokine (C-C motif) ligand 2 (CCL2) and cytokines like interleukin (IL-12), IL-15 and IL-18, which are involved in the recruitment and activation of natural killer (NK) cells. Once activated, NK cells infiltrate neoplastic lesions and kill senescent cancer cells upon the recognition of NKG2D ligands displayed on their surface. These processes result in the progressive elimination of malignant cells and hence inhibit tumor progression. In contrast, Tp53 mutations allow cancer cells to bypass the senescence program and hence form tumors that are not subjected to NK cell-dependent immunosurveillance. Indeed, although p53-deficient malignant cells express near-to-normal levels of NKG2D ligands on their surface, they are unable to efficiently recruit and activate NK cells, which allows tumors to escape immunosurveillance.