Figures & data
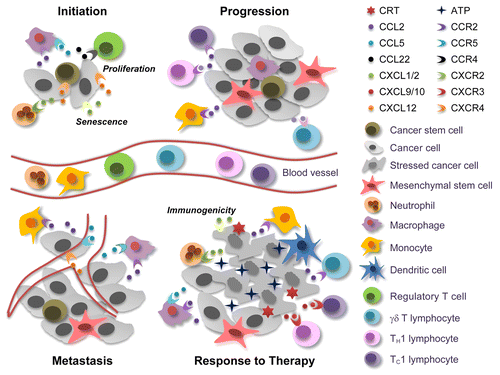
Figure 1. Janus-faced effects of chemokine and chemokine receptors in cancer. At the tumor initiation stage, cancer stem cells (CSCs) can be recruited to favorable niches by chemokine (C-X-C motif) ligand 12 (CXCL12), which signals via chemokine (C-X-C motif) receptor 4 (CXCR4). Macrophages and regulatory T cells are also attracted to these sites by chemokine (C-C motif) ligand 2 (CCL2), CCL5, and CCL22, thus contributing to the establishment of a microenvironment that supports tumor initiation. Conversely, neutrophils, which are attracted to developing neoplastic lesions by CXCL1 or CXCL2 (signaling via CXCR2), can exert tumor-supporting or tumor-suppressing effects, depending on their (N1 or N2) phenotype. CXCL1 and CXCL2 can also promote cell senescence, hence exerting direct antineoplastic effects, while CXCL12 generally accelerate tumor growth. When neoplastic lesions are established, CCR2+ tumor-infiltrating monocytes and tumor-associated macrophages cooperatively support disease progression, driving the abortive activation of immune effector cells and promoting the metastatic dissemination of malignant cells via the CCL5/CCR5 and CXCL12/CXCR4 signaling axes. In response to chemo- or radiotherapy, neoplastic cells die to massive extents. This results in the release of various danger signals including ATP, which is critical for the recruitment and differentiation of antigen-presenting cells. The immune cells that infiltrate neoplastic lesions in response to chemo- or radiotherapy produce high amounts of CCR2 ligands, hence amplifying their own accumulation. Therapy can also trigger the secretion of CXCL1 or CXCL2 from dying tumor cells, resulting in an optimal exposure of the immunogenic factor calreticulin (CRT). Finally, CCL2 can favor the recruitment of interleukin (IL)-17-producing γδ T cells, and the IL-17-dependent release of CXCL9 or CXCL10 promotes the accumulation of interferon γ-secreting CD8+ T cells that mediate tumor clearance.