Figures & data
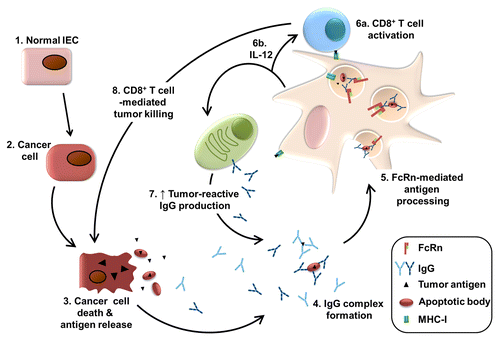
Figure 1. FcRn-mediated antitumor immunosurveillance relies on the cross-priming of CD8+ T cells in the presence of DC-derived IL-12. Normal intestinal epithelial cells (IEC) undergo neoplastic transformation in response to a variety of cues (1,2). The death of neoplastic cells results in the release of tumor-associated antigens (TAAs), either alone (during necrosis) or as part of apoptotic bodies (during apoptosis) (3). Cross-reactive natural IgGs form immune complexes (ICs) with TAAs or tumor-derived apoptotic bodies (4). These ICs are taken up by tumor-infiltrating dendritic cells (DCs), bind to the neonatal Fc receptor for IgG (FcRn) and are routed to an antigen-processing pathway that culminates in the presentation of tumor-derived epitopes onto MHC class I molecules at the cell surface (5). The interaction of naïve CD8+ T cells with the TAA/MHCI molecules results in the activation of tumor-targeting cytotoxic T cells (6a). The cross-linking of FcRn by ICs also leads to the secretion of interleukin-12 (IL-12) by DCs, which promotes the complete activation of CD8+ T cells (6b). IL-12 also stimulates local plasma cells to secrete additional tumor-reactive IgGs (7). The killing of cancer cells by cytotoxic CD8+ T lymphocytes increases the release of TAAs, thereby creating a positive feedback loop enabling potent FcRn-mediated antitumor immunity (8).