Figures & data
Figure 1. Establishment and characterization of an MCA205 cell line expressing tetracycline-inducible Rtn-1c. (A) Vectors used to stably transduce murine fibrosarcoma MCA205 cells to generate tetracycline (TET)-inducible iRTN-1c MCA 205 cells. (B) Western-blot analysis of the expression of reticulon-1 (Rtn-1C) in control conditions (CT) or upon TET treatment for 12 or 24 h. β-actin was used as loading control. A representative experiment is shown. (C‒F) Immunogenic cell death markers of iRTN-1c MCA 205 cells analyzed in response to 0.3 µM TET, 150 µM cisplatin (CDDP), CDDP + TET, 1 µM MTX, or no treatment. (C) Extracellular ATP as measured by luciferin-luciferase assay, (D) Immunofluorescence staining of calreticulin (CRT) exposure to the surface, (E) HMGB1 release from cells as detected by ELISA. (F) Apoptosis assayed by Annexin V detection of externalized phosphatidylserine and secondary necrosis as detected by staining with the vital dye propidium iodide. MTX treatment was used as a positive control. Tetracycline treated samples were compared with their untreated counterpart. Results are reported means ± SEM of triplicates. *P < 0.05, **P < 0.01 (unpaired Student’s t test).
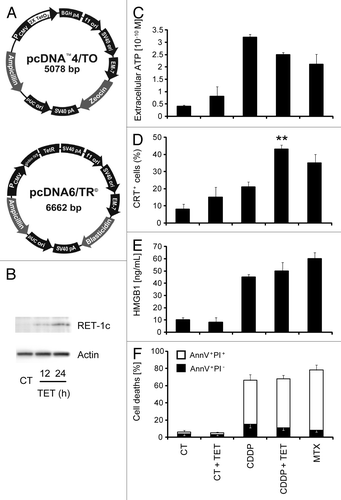
Figure 2. Rtn-1c expression triggers immunogenicity of cell death mediated by CDDP or mitomycin C. (A and B) iRTN-1c MCA205 cells were treated with a single agent or combined with the tetracycline (TET)-induced expression of reticulon-1c (Rtn-1c) and assayed as anticancer vaccines by tumor re-challenge. iRTN-1c MCA205 cells were treated with 150 µM cisplatin (CDDP) or CDDP+ 0.3µM TET (A) or 200 µM mitomycin C (MITOC) or MITOC+ 0.3µM TET (B). 3 x 105 of these dying iRTN-1c MCA205 cells were inoculated s.c. into the left flank of C57BL/6 mice. PBS was injected as a negative control, and mitoxantrone (MTX) treated cells were used as a positive control. One week later, mice were re-challenged with 3 x 104 living MCA205 cells injected s.c. in the contralateral flank, and the absence of tumor growth was scored 60 d later as an indication of an anticancer immune response. The total number of mice for each experiment is indicated (n = 10–15/group) and was obtained by adding independent experiments. Differences between TET-treated groups and their untreated counterparts were analyzed using χ2 test (*P < 0.05).
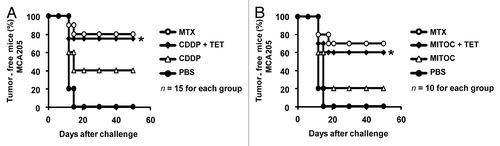
Figure 3. Tumor growth modulation by CDDP and tetracycline treatment in parental and iRTN-1c MCA205 tumors. (A‒D) Tetracycline (TET) mediated reticulon-1c (Rtn-1c) expression combined with cisplatin/CDDP chemotherapy effects the growth rate of established tumors. 2 × 105 parental MCA205 (A and B) or iRTN-1c MCA205 cells (C and D) were injected s.c. into the flank of C57Bl/6 mice. Once palpable, tumors were treated by a single i.p. injection of 0.25 mg/kg CDDP or vehicle (PBS) (day 0) and tumor growth was monitored with a caliper for 25 d. Normal water (A and C) or 100 µM dose TET (B and D) were added in bibber waters for the duration, starting 7 d prior to the cell injection. Moreover, PBS (A and C) or 0.3 µM tetracycline (B and D) were injected intratumorally on day 0. Experiments were done on groups of n = 5 mice and repeated at least twice. Results are reported as means ± SEM *P < 0.05 (unpaired Student’s t test).
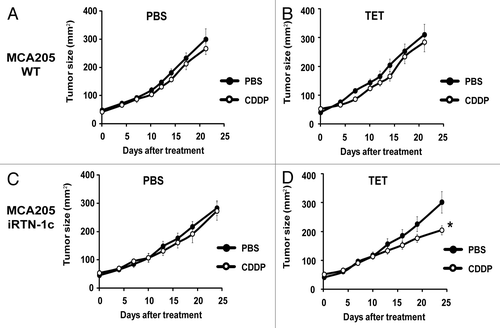
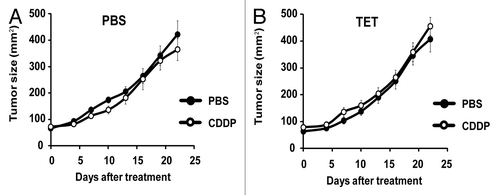
Figure 4. CDDP and tetracycline fail to reduce tumor growth in immunodeficient hosts. (A and B) Tetracycline (TET) mediated reticulon-1c (Rtn-1c) expression combined with cisplatin/CDDP chemotherapy is ineffective against established tumors in athymic nu/nu mice. 2 x 105 iRTN-1c MCA205 cells were injected s.c. into the flank of athymic nu/nu mice. Once palpable, tumors were treated by a single i.p. injection of 0.25 mg/kg cisplatin (CDDP) or vehicle (PBS) (day 0) and tumor growth was monitored with a caliper for 25 d. Normal water (A) or 100 µM dose TET-enriched water (B) were added in bibber bottles for the duration of the experiment, starting 7 d prior to the injection. Moreover, PBS (A) or 0.3 µM tetracycline (B) were injected intratumorally on day 0. Experiments were done on n = 5 mice per group and repeated at least twice. Results are reported as means ± SEM *P < 0.05 (unpaired Student’s t test).