Figures & data
Figure 1. Schematic presentation of the topology of claudin monomer. The model depicts the conserved structural features of claudin and the known interaction sites. ECL1 and ECL2 denote the extracellular loops 1 and 2, respectively. The transmembrane domains 1 to 4 (TM1-TM4) and the regions important for hepatitis C virus (HCV) entry, paracellular ion selectivity, Clostridium perfringens enterotoxin (CPE) binding, and ZO-1 binding are shown.
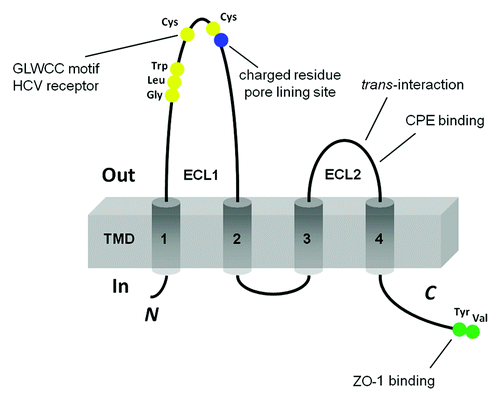
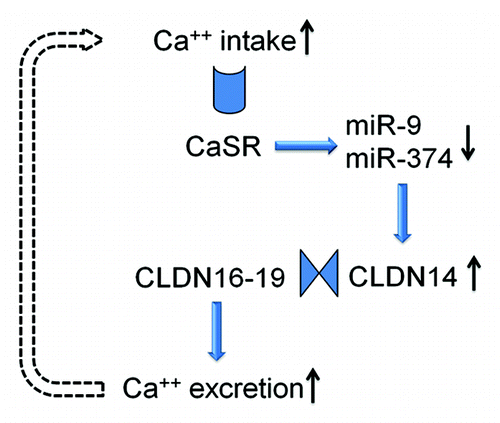
Figure 2. The structural model of paracellular channel. The paracellular channel is depicted as cylinders joining two neighboring cell membranes and allowing selective permeation of cation (Na+) and anion (Cl−) respectively.
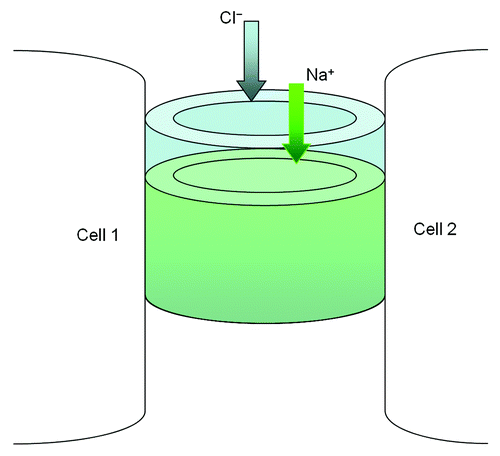
Figure 3. Schematic presentation of interaction possibilities between claudin molecules. The cis interaction includes homomeric or heteromeric interaction; the trans interaction includes homotypic or heterotypic interaction.
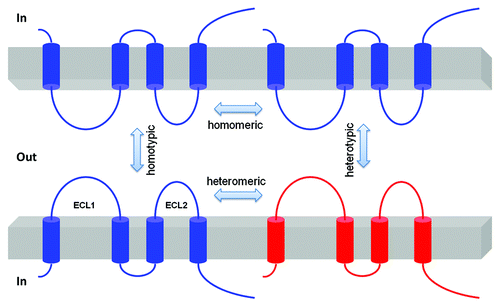
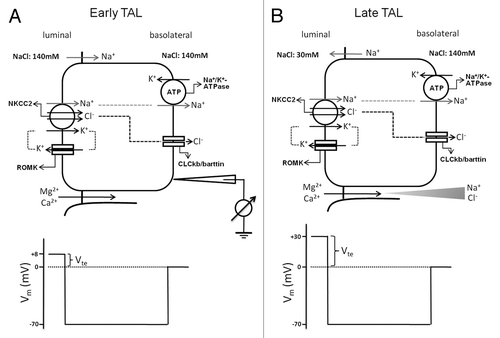
Figure 4. Transepithelial ion transport in the thick ascending limb segment. (A) When similar salt concentrations are present at the luminal and basolateral sides, the luminal spontaneous potential Vte of +8mV is generated by the concerted action of luminal K+ channels, basolateral Cl− channels, the Na+2Cl−K+ cotransporter, and the Na+K+-ATPase. Vte drives Na+ absorption through the paracellular pathway. (B) When a dilute luminal fluid is present after NaCl absorption along the water-tight TAL, the luminal potential Vte of +30 mV is now generated as a diffusion voltage by the ‘backleak’ of Na+. The diffusion voltage depends on the permselectivity of the tight junction. The membrane voltage (Vm) trace depicts the virtual measurement by an electrode that is pushed from the basolateral side through the cell to the luminal side.