Figures & data
Figure 1. Insect Malpighian tubules (A), Malpighi’s original drawing of the MpTs (and gut) of the silkmoth Bombyx mori (Malpighi, 1669). The MpTs are indicated with arrowheads. (B) Schematic representation of the MpTs of Drosophila. Drosophila, has four MpTs, a longer anterior pair and a shorter posterior pair (a single anterior tubule is depicted). Each tubule is divided into four regions based on differences in cell structure and physiology along the proximodistal axis: initial, transitional, main segment and ureter. Two main physiologically distinctive cell types are known, principal cells (yellow) and stellate/bar cells (green). Midgut (MG) and hindgut (HG) are indicated. Image in A reproduced courtesy of the Royal Society of London.
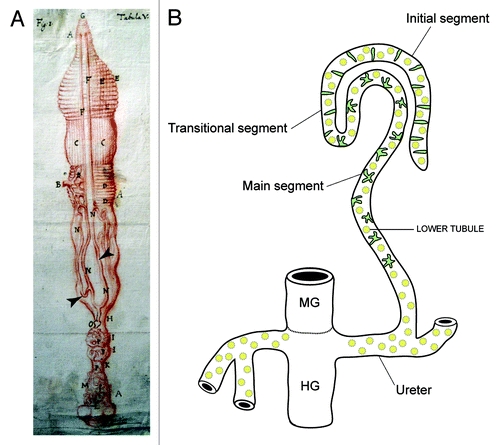
Figure 2. Embryonic development of Drosophila Malpighian tubules A, Stage 11 (5h15–7h15) MpTs grow out as two then four small buds. Cell division occurs synchronously in all tubule cells and then in a subset of tubule cells regulated by Wingless. (B) Stage 12 (7h15–9h15) cell proliferation continues in a subset of tubule cell regulated by the EGF secretion from the tip cell (green cell) and sibling cell (not highlighted). The caudal visceral mesoderm (yellow cells) migrate toward the tubule. (C) Stage 13 (9h15–10h15) Cell division is complete. Stellate cells integrate into the tubule epithelium and develop apico-basal polarity. (D) Stages 13–16 (10h15–13h) Tubule elongation takes place by cell intercalation. MpTs migrate through the body cavity following highly stereotypical routes. (E) Stages 16/17 (13h-22h) the tip cell locates and anchors to its final position [alary muscle (AM) for anterior tubules and hindgut visceral nerve (HVN) for posterior tubules]. The onset of physiological activity becomes apparent as white crystals of uric acid appear in the lumen of the posterior MpTs.
![Figure 2. Embryonic development of Drosophila Malpighian tubules A, Stage 11 (5h15–7h15) MpTs grow out as two then four small buds. Cell division occurs synchronously in all tubule cells and then in a subset of tubule cells regulated by Wingless. (B) Stage 12 (7h15–9h15) cell proliferation continues in a subset of tubule cell regulated by the EGF secretion from the tip cell (green cell) and sibling cell (not highlighted). The caudal visceral mesoderm (yellow cells) migrate toward the tubule. (C) Stage 13 (9h15–10h15) Cell division is complete. Stellate cells integrate into the tubule epithelium and develop apico-basal polarity. (D) Stages 13–16 (10h15–13h) Tubule elongation takes place by cell intercalation. MpTs migrate through the body cavity following highly stereotypical routes. (E) Stages 16/17 (13h-22h) the tip cell locates and anchors to its final position [alary muscle (AM) for anterior tubules and hindgut visceral nerve (HVN) for posterior tubules]. The onset of physiological activity becomes apparent as white crystals of uric acid appear in the lumen of the posterior MpTs.](/cms/asset/0786ede4-6b18-4feb-974f-d0fb0a47c876/kogg_a_10924107_f0002.gif)
Figure 3. Principal and stellate cells (A), Drawing to show the major physiological activities performed by PCs (yellow) and SCs (green) in adult Drosophila tubules. Ion transport is driven by the activity of a H+-transporting vacuolar-ATPase (V-ATPase) located on the luminal membrane of principal cells. The V-ATPase, coupled with a cation/H+ antiporter (NHA), constitutes a cation pump that transports cations from the cytoplasm to the tubule lumen. Chloride ions (black dashed arrows) move into the lumen down an electrochemical gradient possibly through as yet unidentified chloride permeable channels (green) in SCs and via paracellular routes. Water (blue dashed arrows) follows by osmosis through water channels (dark blue) in SCs and via paracellular routes. Possible routes for basolateral cation entry include: K+ channels (orange), cation coupled Cl- cotransporters (light blue), and Na/H exchangers (NHE, black). Movement of ions between cells may occur through gap junctions (GJ). capa, capability peptides 1 and 2; DH, diuretic hormone; LK, leucokinin; LKR, leucokinin receptor; SJ, septate junction; BM, basement membrane. (B) Small section of an adult Drosophila MpT depicting a SC (green) and PCs (unlabelled). PCs and SC are indicated, the tubule is counterstained with phalloidin (red) to highlight the actin cytoskeleton and DAPI (blue) to highlight nuclei.
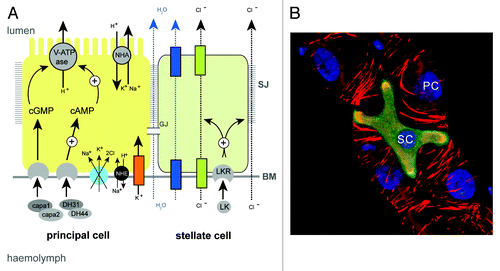
Figure 4. Shaping of the Malpighian tubule (A and B), During a period of about four hours MpTs undergo a dramatic change in shape that is driven by orderly cell intercalation, transforming from short, stubby tubules in mid-embryogenesis stage 13, (A) to long, elongated tubules in late embryogenesis stage 16 (B). (C) SIMI-Biocell assisted 3-D reconstruction of the distal portion of an anterior MpT. Five cells are marked in different colors and their positions tracked in XYZ over time. The distal-most tip cell is marked with red star. Such tracking can be used to quantify individual cell behaviors during tubule elongation (D). (D) Quantification of cell speed during intercalation. Cell speeds for the 5 cells marked in C are shown. The lines show cell tracks and color indicates cell speed. Net direction of cell movement is indicated by the arrow. (E) Orderly cell intercalation fails in crossveinless-c mutants. Anterior and posterior MpTs are indicated by yellow and cyan arrowheads in A, B and E. Images in C and D courtesy of Aditya Saxena.
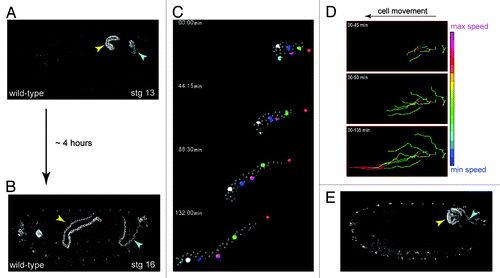
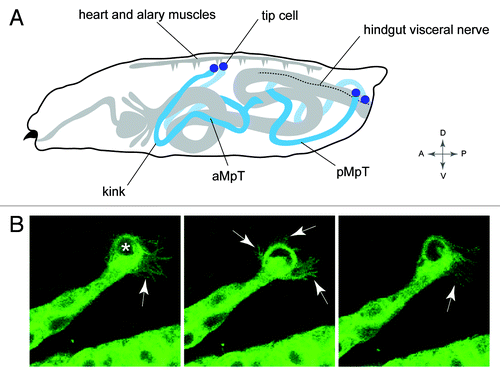
Figure 5. Positioning the tubule in the body cavity (A), Drawing of a Drosophila larva showing the final position of the MpTs relative to other internal structures. Anterior and posterior MpTs (aMpT and pMpT) are indicated, tip cells are shown in purple, gut and heart are shown in gray, the hindgut visceral nerve is depicted by a dashed line. Anterior, posterior, dorsal, ventral coordinates are given. (B) Still images from MpT movie showing the dynamic filopodial extensions (arrows) of the tip cell (indicated in first panel by asterisk). The time interval between images is 5 min. Images in B courtesy of Helen Weavers.