Figures & data
Table 1. Neurofiliament and peptide content of DRG neurones with identified C, Aδ or Aα/β fiber conduction velocities (data extracted from ref. Citation36)
Figure 1. The components of afferent noise in the guinea pig. (A) Illustrates the broad elements of the afferent systems associated with the bladder: components of afferent noise. Four are identified: pain, mechano-sensory, urothelial and motor-sensory. Each sends afferent information to the CNS but only the motor-sensory system has the potential for an output from the CNS and inputs from peripheral afferent fibers. (B) Illustrates in more detail some of the component parts of the systems making up afferent noise. The afferent out flow to the CNS can again be seen for each system: 1, pain; 2, mechano-sensitive (stretch); 3, urothelial; 4, motor-sensory. For the motor-sensory system, part of the complex regulatory systems involved in the regulation of motor activity may be occurring via the intra-mural ganglia and local neural circuits within the bladder wall. Reprinted from reference Citation43 with permission.
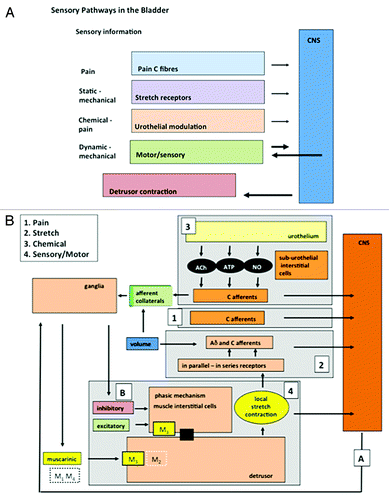
Figure 2. Motor activity and the generation of afferent firing. (A) Illustrates one of the original records of non-voiding motor activity recorded in the cat from where it was first proposed that this activity was associated with afferent firing and the control of micturition. (B) Illustrates confirmation of this concept showing the motor activity in the cat (upper record) with associated bursts of afferent nerve activity (lower record) (reprinted from reference Citation58 with permission). (C) Shows a cystometric record from a conscious rat during bladder filling. Voids can be seen at the beginning and end of the recording. During the filling phase small non-voiding contractions appear, progressively increasing in amplitude and frequency as the bladder fills. The progressive increase in motor activity implies that the intensity of afferent activity is also increasing during filling (reprinted from reference Citation59 with permission).
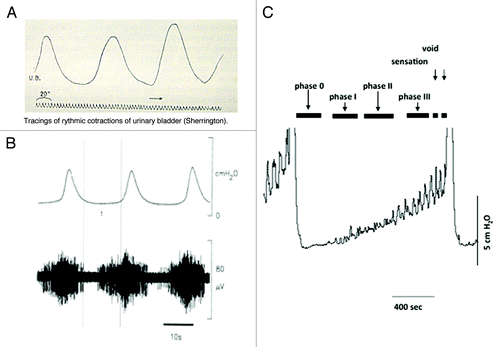
Figure 3. The effects of the β3 specific agonist mirabegron on motor sensory noise in the conscious partially obstructed rat (modifed from reference Citation10). (A) Illustrates an original record showing 4 filling and voiding cycles during cystometry in a conscious rat. The micturition contractions are easily seen. The first 2 cycles are under control conditions and the motor component of the motor-sensory system, the non-voiding activity, is apparent. This is more clearly seen in section (B) where the non-voiding activity has been isolated by filtering. The β3 specific agonist (YM-178, mirabegron) was then added and the effects on the following filling and voiding cycles noted. The drug clearly affects the non-voiding activity but has little effect on the amplitude of the voiding contraction. (Reprinted from reference Citation10 with permission). (B) Shows a cartoon proposing how on-voiding activity and micturition activity is generated in the rat. The accepted parasympathetic motor system is there to initiate the large voiding contraction. In addition the system generating and modulating the motor component of the motor-sensory noise behaves as though it had a “pacemaker” controlled by cholinergic (excitatory) and adrenergic (inhibitory) inputs. Afferent fibers (green) respond to the local contractions and stretches sending information related to bladder volume to the CNS.
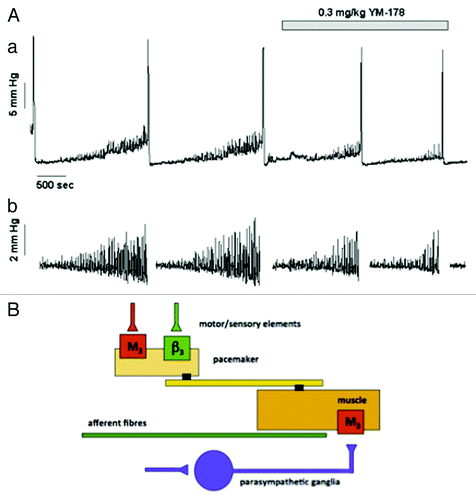
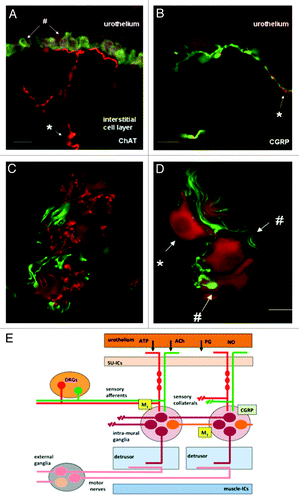
Figure 4. Images of afferent nerves and intra-mural ganglia in the guinea pig bladder. The sections were stained for choline-acetyltransferase (ChAT) (red) detecting the enzyme responsible for synthesising acetylcholine. The sections were also stained with an antibody to calcitonin gene related peptide (CGRP) (green). Almost certainly these CGRP fibers are afferent fibers. (A and B) Show images of sub-urothelial cholinergic (A) and peptidergic (CGRP) (B) nerves indicating two distinct populations of afferent fiber. (C and D) Show cholinergic terminals (C, red) within the intra-mural-ganglia as are CGRP terminals (C and D, green). These observations suggest the possibility of integration of different inputs into these neurons. Panel E shows a cartoon illustrating the possible interactions of afferent collaterals, both peptidergic (CGRP) and cholinergic with the intra-mural ganglia. An output to the muscle is postulated that is in addition to the conventional parasympathetic system involved in the initiation of the voiding contraction (reprinted from reference Citation73 with permission).