Figures & data
Figure 1. Isolation and primary culture of human ADSCs. The isolated process and primary culture of human ADSCs were described in the Materials and Methods. (A) The pieces of adipose tissue attached on the walls of culture flasks on the first day of culture. (B) The ADSCs demonstrated spindle-like morphology on the seventh day of culture. (C) The ADSCs with fibroblast-like morphology developed into 80–90% confluence after 10 d of primary culture. (D) One representative colony with sphere-like shape was observed in 2-week culture. Scale bars: 200 µm
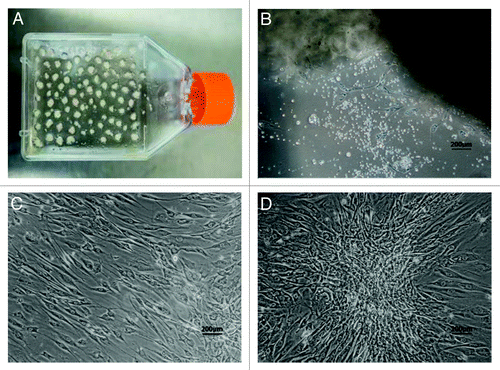
Figure 2. Cell surface antigens of human ADSCs were detected by flow cytometry. The ADSCs at passage 3 were processed with FITC or PE-conjugated monoclonal antibodies to test a set of expression levels of cell surface antigens as described in the Materials and Methods. Nonspecific IgG were examined as a control (red). (A) Representative results of flow cytometry analysis, which showed that ADSCs could expressed positively CD44, CD105, CD29, CD90, and CD13. In contrast, almost no expression of the hematopoietic lineage markers of CD45, CD34, CD31 and CD106 was detected in ADSCs. (B) The geometric mean of cell surface antigens detected by flow cytometry.
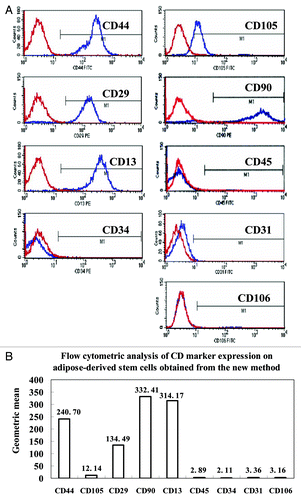
Figure 3. Adipogenic and osteogenic differentiation of human ADSCs in vitro. Human ADSCs at passage 2 were cultured in the induction medium for two weeks for adipogenic differentiation, or for three weeks for osteogenic differentiation. (A) Adherent ADSCs, no staining as a negative control for adipogenic differentiation. (B) Oil red-O staining for induced ADSCs was positive, and presence of lipid droplets demonstrated that ADSCs could differentiate into adipogenic lineage. (C) Adherent ADSCs, no staining as a negative control for osteogenic differentiation. (D) Von Kossa staining showed that a calcified extracellular matrix of induced ADSCs was detected, which confirmed ADSCs could differentiate into osteogenic cells. Scale bars: 200 µm
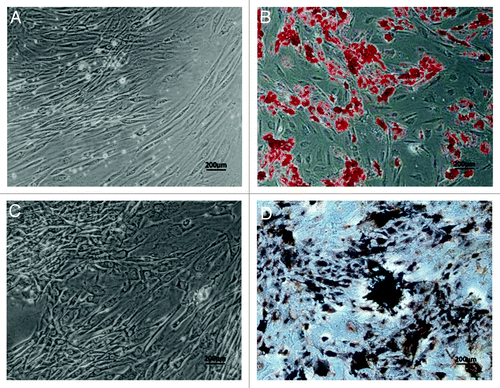
Figure 4. Growth kinetics and senescence of human ADSCs were assayed. The human ADSCs at passage 3 were inoculated in 96-well microplates with 100 μL of cell suspension at a density of 2000 cells/well, and its proliferative potential was measured using cell counting kit-8. (A) The results of cell proliferation were delineated as a growth curve, which showed ADSCs could be amplified and maintained for an extended culture periods. (B and C) Cellular senescence was assayed by staining ADSCs at passage 8 and 15 for β-Gal expression, respectively. The arrows in the photographs indicated β-Gal staining positive cells that were senescent cells, and percentage of cellular senescence at passage 8 (B) and 15 (C) was both below 5%. These results demonstrated that ADSCs could maintain a stable long-term culture period without senescence in vitro. Scale bars: 100 µm
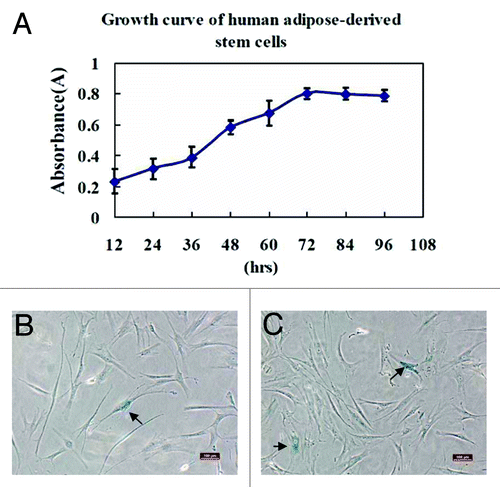
Figure 5. Colony formation of human ADSCs was assayed. The clonogenic ability of primary ADSCs on the sixth day was analyzed by seeding cells in 35 mm dishes at an indicated cell density for two weeks of culture. Formed colonies were stained with crystal violet solution. Colony forming unit (CFU) containing at least ten cells was defined as a colony and counted under a microscope. (A) 100 cells/dish; (B) 300 cells/dish; (C) 400 cells/dish; (D) The quantities of CFU showed that colony formation of ADSCs increased in a cell concentration-dependent manner. Values were presented as means ± SD (n = 3). *P < 0.05 group B or C vs. group A.
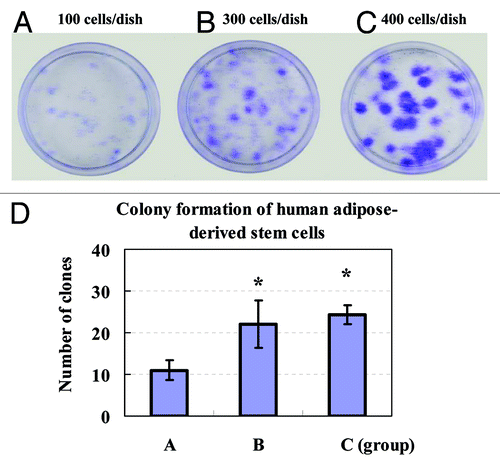
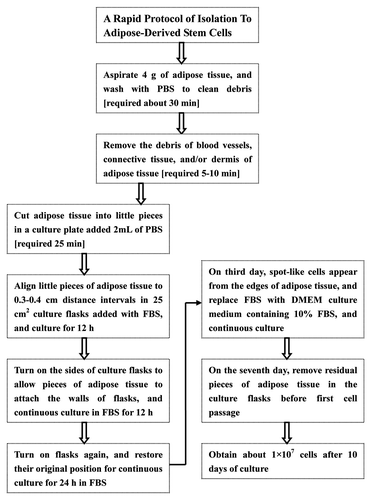
Figure 6. Isolation procedure of human ADSCs with technique flowcharts. No centrifugation and collagenase digestion are required in our isolation protocols for ADSCs from human adipose tissue, and multiple steps and intensive efforts are also decreased, but large numbers of ADSCs can be harvested by this new method.