Figures & data
Figure 1. Metakaryotic nuclei undergoing two forms of symmetrical amitoses within tubular syncytia of fetal spinal cord ganglia (9 wks). (A, B, and C) Three “kissing bell” amitoses with DNA stained Feulgen reagent (purple). The order shown represents the stages of (A) formation of two pairs of condensed DNA rings at the rim of the bell mouth that (B) separate as total DNA increases and (C) proceeds toward complete separation when an exact doubling of DNA is observed.Citation3 (D, E, and F) Three “kissing bell” amitoses stained with acridine orange after treatment with RNase. The order shown represents the stages of (D) appearance of bright orange fluorescence co-localized with the condensed DNA rings of Feulgen stained condensed DNA as in (A), (E) separation of two orange rings at the bell mouths as total DNA increases as in (BandF) progress toward complete separation as in (C). (G, H, I, and J) Four lower magnification images of syncytia stained with acridine orange after RNase treatment. (G) A section of a syncytium with well-separated bell shaped nuclei showing the common “stacked cup” relationship of metakaryotic nuclei in tubular syncytia when no amitoses are evident. Well-separated bell shaped nuclei stained with acridine orange emit green fluorescence with scant amount of orange fluorescence. (H) A tubular syncytial section with bell shaped nuclei displaying the closely stacked nuclei indicative of synchronous amitoses and strong orange fluorescence associated in this example with the rims of the bell mouths. (I and J) Two syncytial sections displaying the “stacking cup” form of amitoses with varying degrees of separation between nuclei and the intensity and distribution of orange fluorescence among nuclei within the syncytia. Images by EVG.
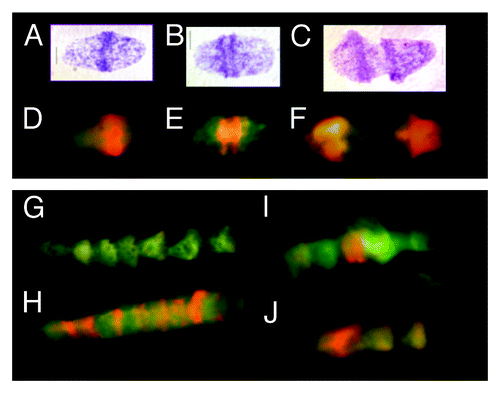
Figure 2. Fluorescent (FITC green) Mab F7–26 ssDNA antibody complex and acridine orange staining of RNase-treated syncytia within tubular syncytia of fetal spinal cord ganglia (9 wks). (A, C, and E) Acridine orange staining showing various spatial distributions of bright orange fluorescence in syncytial bell shaped nuclei. (B, D, and F) Anti-ssDNA labeling (FITC green) of bell shaped nuclei in the same samples with DAPI (blue) staining of dsDNA. Arrows emphasize the similarity of distribution of both modes of ssDNA recognition starting at the rim of the mouths of the nuclei. Scale bar, 5 µm. Images by Gostjeva EV, Fomina JN, and Darroudi F.
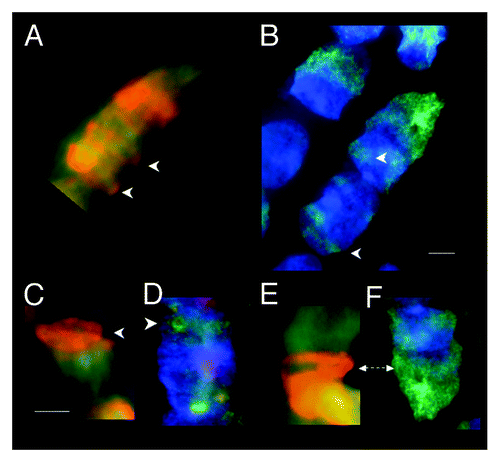
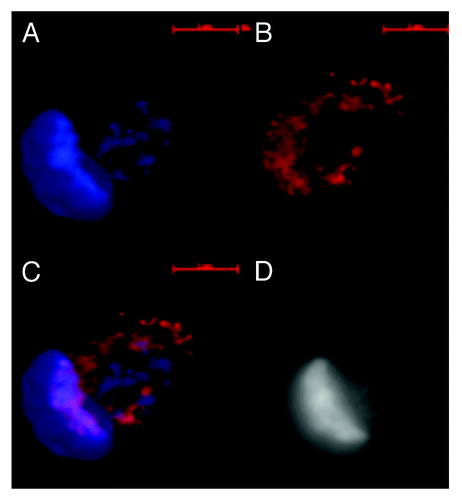
Figure 3. Images of dsDNA (DAPI, blue) and dsRNA/DNA (TRITC-Ab, red) stain in metakaryotic syncytial nuclei undergoing symmetric amitoses in fetal spinal cord, 10 wks. (A) DAPI fluorescence (blue). (B) TRITC-Ab fluorescence (red). (C) Merged images of (A) and (B) showing nuclei labeled simultaneously with DAPI and TRITC-Ab-n3. (D) Achromatic image of (A). Scale bar, 5 µm. Image by Koledova VV.
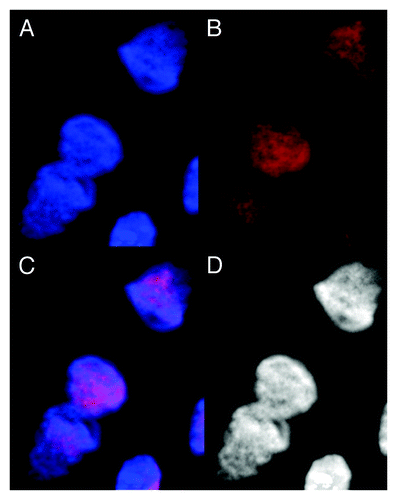
Figure 4. Images of dsDNA (DAPI, blue) and dsRNA/DNA (TRITC-Ab, red) stain in metakaryotic syncytial nuclei undergoing synchronous symmetrical amitoses in fetal spinal cord, 10 wks. (A) DAPI fluorescence (blue). (B) TRITC-Ab fluorescence (red). (C) Merged images of (A) and (B) showing nuclei labeled simultaneously with DAPI and TRITC-Ab. (D) Achromatic image of (A). Image by Koledova VV.
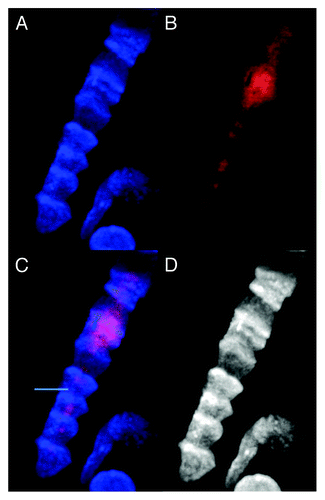
Figure 5. Images of dsDNA (DAPI, blue) and dsRNA/DNA (TRITC-Ab, red) stain in mononuclear metakaryotic cell undergoing asymmetrical amitosis in a colonic adenocarcinoma (M, 68 y). (A) DAPI fluorescence (blue). (B) TRITC-Ab fluorescence (red). (C) Merged images of (AandB) showing nuclei labeled simultaneously with DAPI and TRITC-Ab. (D) Achromatic image of (A). Image is interpreted as an asymmetrical amitosis in which both parent bell shaped nucleus and daughter spherical nucleus have reconverted a large fraction of the dsRNA/DNA intermediate (red) to the dsDNA form (blue). Appearance of red fragments or striations of dsRNA/DNA antibody labeling in cytoplasmic volume between nuclei is occasionally observed as shown here in tumors and in the HT-29 cell line. Image by Koledova VV.
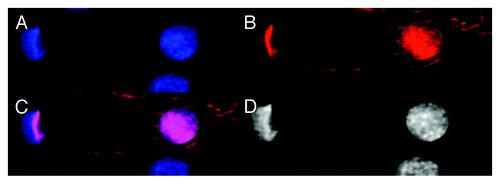
Figure 6. Image of dsDNA (DAPI, blue) and dsRNA/DNA (TRITC-Ab, red) in mononuclear metakaryotic nucleus undergoing asymmetrical amitosis in the HT-29 cell line. (A) DAPI fluorescence (blue). (B) TRITC-Ab fluorescence (red). (C) Merged images of (AandB) showing nuclei labeled simultaneously with DAPI and TRITC-Ab. (D) Achromatic image of (A). This figure is interpreted pro tempore as an asymmetric form of amitosis: parent bell shaped nucleus has been reconverted to dsDNA (blue) without any indication of dsRNA/DNA (red) but in the derived oval nucleus a substantial fraction of the dsRNA/DNA intermediate (red) has been reconverted to dsDNA (blue) during nuclear segregation which is not yet completed in this example. Asymmetrical cell division is a characteristic expected of a stem cell. Image by Koledova VV.