Figures & data
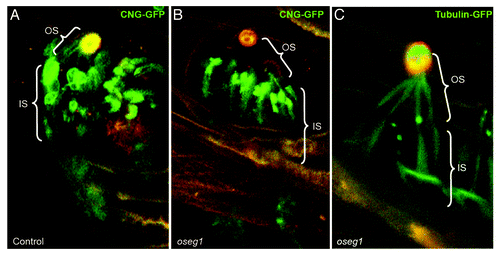
Figure 1. The ciliary transition zone and related transport pathways. (A) The cilium is a hair like protrusion that extends from the cell surface. It consists of the centriole (basal body) that is located in the cytoplasm, the axoneme, which forms as an extension of centriolar microtubules, and the membrane that encases the axoneme. Several structures associate with the base of the cilium: the ciliary pocket, an invagination found at the junction of the plasma membrane and cilium membrane; the septin ring, a protein scaffold that associates with the cilium periphery; transition fibers, which are appendages that connect the distal centriole to the cell membrane; and the transition zone (TZ), which is a specialized and structurally distinct region found between the centriole and the cilium proper. (B) Key proteins that mediate and regulate transport into the ciliary compartment. Proteins destined for cilia are delivered via several routes. Transmembrane proteins are delivered from the Golgi apparatus in vesicles, which travel through the cytoplasm toward the cilium and fuse with the ciliary or periciliary membrane. Several protein complexes and pathways have been identified to function in this process (boxes). The transmembrane Smoothened (smo) protein is an unusual case because it is first deposited in the plasma membrane, from where it translocates into the cilium upon the activation of the hedgehog signaling cascade. Diffusion is likely to be the driving force that translocates cytosol-soluble proteins toward the cilium base. A subset of lipidated proteins is also solubilized in the cytoplasm by UNC119. Despite their hydrophobicity, these proteins are likely to behave similarly to cytoplasmic proteins. Inside the ciliary shaft, proteins are translocated via a mechanism known as intraflagellar transport (IFT).
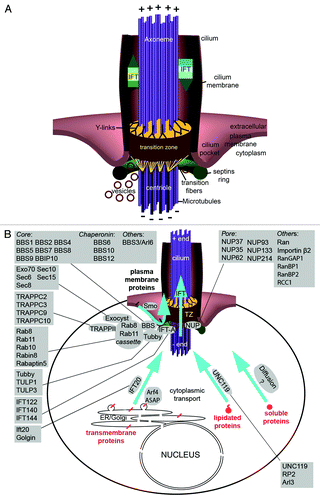
Figure 2. Transition zone protein modules. (A–C) Transition zone proteins classified into MKS (Meckel-Gruber Syndrome) module components (A), NPHP (Nephronophthisis) module constituents (B) and others that are not currently categorized (C). Some of the proteins, when mutated, lead to other syndromic diseases such as Joubert syndrome (JBTS). (D) The transition zone protein Cep290 is conserved in both of the main branches of eukaryotic evolution.Citation51,Citation55,Citation199
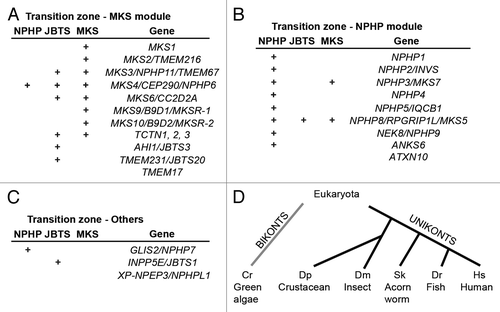
Figure 3. Specialized transition zones. (A) Vertebrate photoreceptors and Drosophila sensory neurons share similar cellular architecture and an atypical transition zone, known as the connecting cilium. The outer segments of vertebrate photoreceptors measure 20–25 um in rods and 10–15 um in cones of most species.Citation200-Citation202 Outer segments of fly sensory neurons are approximately 5–50 um long.Citation203,Citation204 Modified from Avidor-Reiss et al., 2004. (B and C) Illustration of compartmentalized (B) and cytoplasmic (C) ciliogenesis, featuring transition zone and ring centriole/annulus, respectively.
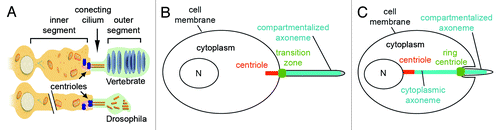
Figure 4. Oseg1/ IFT122 function in ciliogenesis. An IFT Complex A subunit, Oseg1/ IFT122, is essential for CNG channel transport into the sensory cilium. (A) In control Drosophila larvae, the sensory neurons that innervate olfactory organs (autofluorescent in orange) express CNG-Channel-GFP in both the inner (IS) and outer segment (OS). (B) In contrast to that, in oseg1/ift122 mutant larvae, the CNG-Chanel-GFP is only found in the inner segment. (C) This is a specific defect in CNG transport as Tubulin-GFP is found in both the inner and the outer segment of oseg1 mutants. Avidor-Reiss Lab, unpublished results.