Figures & data
Figure 1 Canonical Wnt/β-catenin signaling. In the absence of Wnt signals (left), cytoplasmic β-catenin protein (β) is bound by a “destruction complex” of proteins that include APC and Axin, both of which are encoded by tumor suppressor genes, and the serine/threonine-kinase GSK3. Phosphorylation of β-catenin by GSK3 targets it for ubiquitination and degradation. Wnt protein binding to the Frizzled and LRP5/6 co-receptors (right) activates cytoplasmic Disheveled protein (Dsh), which disrupts the destruction complex, possibly by recruitment of Axin, thus permitting accumulation of unphosphorylated β-catenin. As β-catenin levels increase, a fraction of the cytoplasmic pool translocates to the nucleus and associates with DNA-binding proteins of the TCF family, displacing Groucho repressor proteins (Gro, left) and activating expression of downstream target genes such as cMyc. In epithelial cells, β-catenin also associates with cadherin proteins at the cell surface (not shown here), potentially stabilizing cell-cell interactions.Citation6
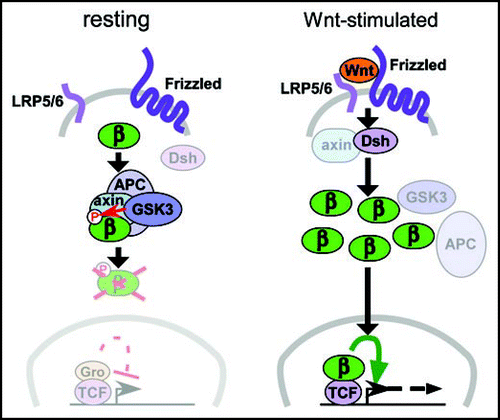
Figure 2 Stages of pancreatic organogenesis. The dorsal and ventral pancreata (left, dp and vp) are specified in the foregut between embryonic days 8.5–9.5 (E8.5–E9.5) in the mouse, near the forming liver (li). Over the next several days, the dorsal and ventral primordia initiate a program of rapid growth and branching morphogenesis (center), eventually producing lobular organs that lie adjacent to the stomach (st) and intestine (in), respectively. A massive wave of differentiation occurs coincident with the later portion of the growth phase (right), producing a branched ductal network (du) terminated by digestive enzyme-secreting acinar cells (ac), with endocrine islets (is) embedded in the interstitial space. As described in the text, Wnt/β-catenin signaling appears to regulate multiple steps in this process, including inhibition of pancreas specification, promotion of growth and acinar differentiation, and possibly inhibition of endocrine development.
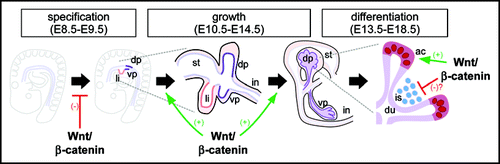
Table 1 Experimental evidence of Wnt/β-catenin function in pancreas development