Figures & data
Figure 1 The three major roles of β-catenin in liver physiology. Left: in the presence of Wnt, β-catenin is released from its inactivating complex and translocates to the nucleus, where it activates genes essential for proliferation, growth and regeneration of the liver. Middle: β-catenin mediates cell-cell adhesion through its interaction with e-cadherin on the hepatocyte membrane. Right: in the presence of HGF, β-catenin, which associates with Met at the surface of hepatocytes, is phosphorylated and translocates to the nucleus to turn on genes important in proliferation and morphogenesis.
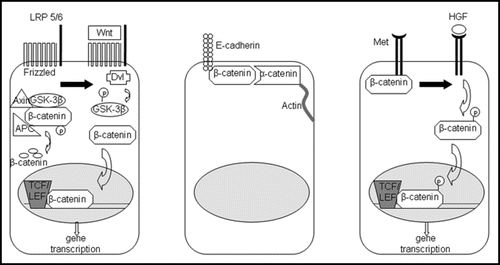
Figure 2 Model of embryonic liver development. Signals such as FGF-1, FGF-2 and BMP4 emanating from the cardiac mesoderm specify the foregut endoderm to begin expressing liver-specific genes. The Foxa transcription factors are also required for foregut endoderm specification. The resulting hepatoblasts, which are albumin and a-fetoprotein positive, proliferate and expand. Hepatoblasts express transcription factors such as Hex, Hlx and Prox1, which are essential for liver proliferation and differentiation. HNF-6, HNF-1β and the Notch/Jagged signaling pathway induce differentiation toward a biliary epithelial lineage, while HNF-4α followed by C/EBPα produces mature hepatocytes.
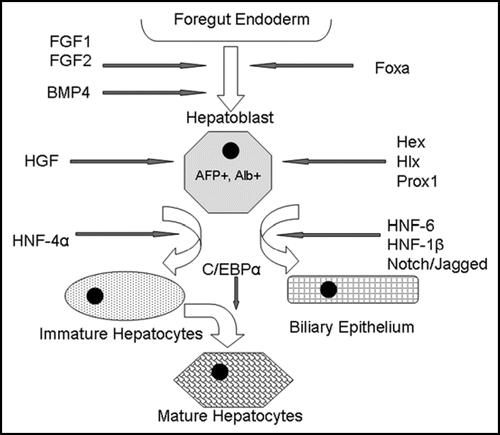
Figure 3 Role of β-catenin in embryonic liver development. β-catenin expression must be suppressed during the competence and specification stage in order for normal liver development to occur. However, expression of β-catenin is essential during the later stages of liver development, such as outgrowth, expansion and differentiation. Although there is conflicting evidence concerning the role of β-catenin during very early liver development, we hypothesize that β-catenin expression is necessary for hepatic induction immediately after its repression during the competence/specification stage. Thus, β-catenin has been found to play a role in all stages of embryonic liver development.
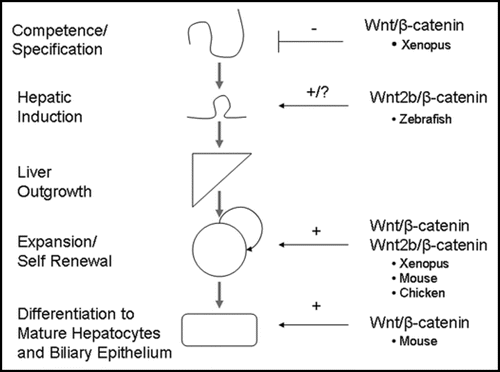
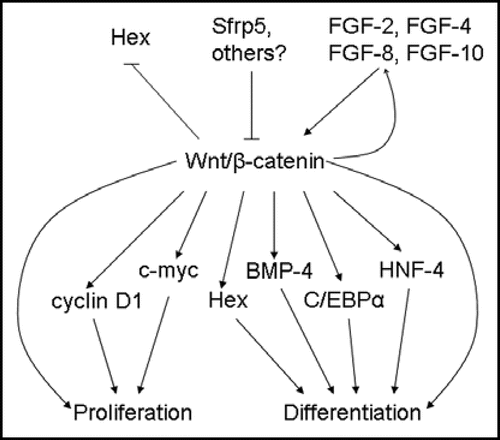
Figure 4 Upstream regulators and downstream targets of β-catenin during embryonic liver development. β-catenin expression in early foregut development inhibits Hex expression; later in hepatogenesis, β-catenin is thought to regulate proliferation in part through Hex. Srfp5 inhibits β-catenin activity, while FGFs stimulate the β-catenin pathway. In turn, β-catenin activates FGF-8 expression, suggesting a feed-forward mechanism. Downstream targets of β-catenin, such as cyclin D1 and c-myc, induce proliferation, while others, such as Hex, BMP4, C/EBPα and HNF-4, promote differentiation.